Cytoskeletal actin gates a Cl- channel in neocortical astrocytes
- PMID: 9464993
- PMCID: PMC2712127
- DOI: 10.1523/JNEUROSCI.18-05-01679.1998
Cytoskeletal actin gates a Cl- channel in neocortical astrocytes
Abstract
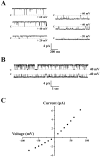
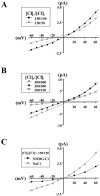
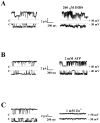
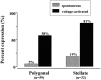
Increases in astroglial Cl- conductance accompany changes in cell morphology and disassembly of cytoskeletal actin, but Cl- channels underlying these conductance increases have not been described. We characterize an outwardly rectifying Cl- channel in rodent neocortical cultured astrocytes and describe how cell shape and cytoskeletal actin modulate channel gating. In inside-out patch-clamp recordings from cultured astrocytes, outwardly rectifying Cl- channels either were spontaneously active or inducible in quiescent patches by depolarizing voltage steps. Average single-channel conductance was 36 pS between -60 and -80 mV and was 75 pS between 60 and 80 mV in symmetrical (150 mM NaCl) solutions. The permeability ratio (PNa/PCl) was 0.14 at lower ionic strength but increased at higher salt concentrations. Both ATP and 4, 4-diisothiocyanostilbene-2,2'-disulfonic acid produced a flicker block, whereas Zn2+ produced complete inhibition of channel activity. The frequency of observing both spontaneous and inducible Cl- channel activity was markedly higher in stellate than in flat, polygonally shaped astrocytes. In addition, cytoskeletal actin modulated channel open-state probability (PO) and conductance at negative membrane potentials, controlling the degree of outward rectification. Direct application of phalloidin, which stabilizes actin, preserved low PO and promoted lower conductance levels at negative potentials. Lower PO also was induced by direct application of polymerized actin. The actions of phalloidin and actin were reversed by coapplication of gelsolin and cytochalasin D, respectively. These results provide the first report of an outwardly rectifying Cl- channel in neocortical astrocytes and demonstrate how changes in cell shape and cytoskeletal actin may control Cl- conductance in these cells.
Figures





References
-
- Cantiello HF, Stow J, Prat AG, Ausiello DA. Actin filaments control epithelial Na channel activity. Am J Physiol. 1991;261:C882–C888. - PubMed
-
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. - PubMed
-
- Colquhoun D, Hawkes AG. On the stochastic properties of single ion channels. Proc R Soc Lond [Biol] 1981;211:205–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
