Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells
- PMID: 9464994
- PMCID: PMC6792611
- DOI: 10.1523/JNEUROSCI.18-05-01693.1998
Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells
Abstract
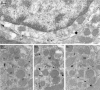
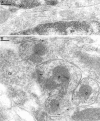
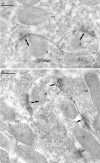
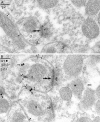
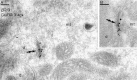
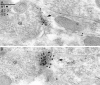
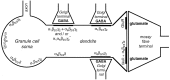
Two types of GABAA receptor-mediated inhibition (phasic and tonic) have been described in cerebellar granule cells, although these cells receive GABAergic input only from a single cell type, the Golgi cell. In adult rats, granule cells express six GABAA receptor subunits abundantly (alpha1, alpha6, beta2, beta3, gamma2, and delta), which are coassembled into at least four to six distinct GABAA receptor subtypes. We tested whether a differential distribution of GABAA receptors on the surface of granule cells could play a role in the different forms of inhibition, assuming that phasic inhibition originates from the activation of synaptic receptors, whereas tonic inhibition is provided mainly by extrasynaptic receptors. The alpha1, alpha6, beta2/3, and gamma2 subunits have been found by immunogold localizations to be concentrated in GABAergic Golgi synapses and also are present in the extrasynaptic membrane at a lower concentration. In contrast, immunoparticles for the delta subunit could not be detected in synaptic junctions, although they were abundantly present in the extrasynaptic dendritic and somatic membranes. Gold particles for the alpha6, gamma2, and beta2/3, but not the alpha1 and delta, subunits also were concentrated in some glutamatergic mossy fiber synapses, where their colocalization with AMPA-type glutamate receptors was demonstrated. The exclusive extrasynaptic presence of the delta subunit-containing receptors, together with their kinetic properties, suggests that tonic inhibition could be mediated mainly by extrasynaptic alpha6beta2/3delta receptors, whereas phasic inhibition is attributable to the activation of synaptic alpha1beta2/3gamma2, alpha6beta2/3gamma2, and alpha1alpha6beta2/3gamma2 receptors.
Figures

References
-
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. - PubMed
-
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
-
- Baude A, Nusser Z, Molnar E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Benke D, Honer M, Michel C, Mohler H. GABAA receptor subtypes differentiated by their γ-subunit variants: prevalence, pharmacology, and subunit architecture. Neuropharmacology. 1996;35:1413–1423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
