Ectopic overexpression of engrailed-2 in cerebellar Purkinje cells causes restricted cell loss and retarded external germinal layer development at lobule junctions
- PMID: 9465001
- PMCID: PMC6792618
- DOI: 10.1523/JNEUROSCI.18-05-01763.1998
Ectopic overexpression of engrailed-2 in cerebellar Purkinje cells causes restricted cell loss and retarded external germinal layer development at lobule junctions
Abstract
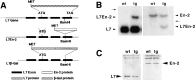
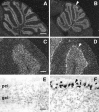
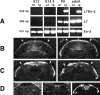
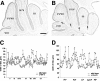
Members of the En and Wnt gene families seem to play a key role in the early specification of the brain territory that gives rise to the cerebellum, the midhindbrain junction. To analyze the possible continuous role of the En and Wnt signaling pathway in later cerebellar patterning and function, we expressed En-2 ectopically in Purkinje cells during late embryonic and postnatal cerebellar development. As a result of this expression, the cerebellum is greatly reduced in size, and Purkinje cell numbers throughout the cerebellum are reduced by more than one-third relative to normal animals. Detailed analysis of both adult and developing cerebella reveals a pattern of selectivity to the loss of Purkinje cells and other cerebellar neurons. This is observed as a general loss of prominence of cerebellar fissures that is highlighted by a total loss of sublobular fissures. In contrast, mediolateral patterning is generally only subtly affected. That En-2 overexpression selectively affects Purkinje cells in the transition zone between lobules is evidenced by direct observation of selective Purkinje cell loss in certain fissures and by the observation that growth and migration of the external germinal layer (EGL) is selectively retarded in the deep fissures during early postnatal development. Thus, in addition to demonstrating the critical role of Purkinje cells in the generation and migration of granule cells, the heterogeneous distribution of cellular effects induced by ectopic En expression suggests a relatively late morphogenetic role for this and other segment polarity proteins, mainly oriented at lobule junctions.
Figures



References
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. III. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–294. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC; New York: 1997.
-
- Baader SL, Schilling ML, Rosengarten B, Pretsch W, Teutsch HF, Oberdick J, Schilling K. Purkinje cell lineage and the topographic organization of the cerebellar cortex: a view from X inactivation mosaics. Dev Biol. 1996;174:393–406. - PubMed
-
- Bian F, Chu T, Schilling K, Oberdick J. Differential mRNA transport and the regulation of protein synthesis: selective sensitivity of Purkinje cell dendritic mRNAs to translational inhibition. Mol Cell Neurosci. 1996;7:116–133. - PubMed
-
- Danielian PS, McMahon AP. Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
