Intrastriatal mesencephalic grafts affect neuronal activity in basal ganglia nuclei and their target structures in a rat model of Parkinson's disease
- PMID: 9465005
- PMCID: PMC6792620
- DOI: 10.1523/JNEUROSCI.18-05-01806.1998
Intrastriatal mesencephalic grafts affect neuronal activity in basal ganglia nuclei and their target structures in a rat model of Parkinson's disease
Abstract
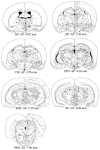
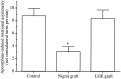
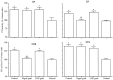
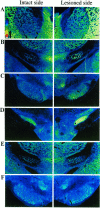
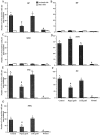
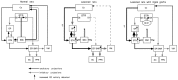
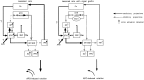
Nigrostriatal dopamine (DA) lesions lead to changes of neuronal activity in basal ganglia nuclei such as the globus pallidus (GP, the rodent homolog of lateral globus pallidus), entopeduncular nucleus (EP, the rodent homolog of medial globus pallidus), substantia nigra pars reticulata (SNR), and subthalamic nucleus (STN). We investigated in rats whether embryonic mesencephalic DA neurons grafted in the striatum may affect the lesion-induced alterations of neuronal activity in these structures. Regional neuronal activity was determined by use of quantitative cytochrome oxidase histochemistry. It was also examined in lesioned rats whether the grafts may regulate the expression of c-Fos after systemic administration of apomorphine in the basal ganglia nuclei as well as their target structures, including the ventromedial thalamic nucleus (VM), superior colliculus (SC), and pedunculopontine nucleus (PPN). Lesioned rats exhibited an increased activity of CO in the GP, EP, SNR, and STN ipsilateral to the lesion. Intrastriatal nigral grafts reversed the increases in the CO activity in the EP and SNR, whereas the grafts failed to affect the enzyme activity in the GP or STN. Apomorphine induced an increased expression of c-Fos in the GP, STN, VM, SC, and PPN on the lesioned side. The enhanced expression of this protein in all the structures except for the STN was attenuated by nigral grafts. The present results indicate that intrastriatal DA neuron grafts can normalize the lesion-induced changes of neuronal activity in the output nuclei of the basal ganglia as well as their target structures.
Figures


References
-
- Abercrombie M. Estimation of nuclear population from microtome section. Anat Rec. 1946;94:239–247. - PubMed
-
- Afsharpour S. Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol. 1985;236:14–28. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Berger B, Thierry AM, Tassin JP, Moyne MA. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Res. 1976;106:133–145. - PubMed
-
- Björklund A. Dopaminergic transplants in experimental parkinsonism: cellular mechanisms of graft-induced functional recovery. Curr Opin Neurobiol. 1992;2:683–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
