Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter
- PMID: 9465024
- PMCID: PMC19012
- DOI: 10.1073/pnas.95.4.1387
Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter
Abstract
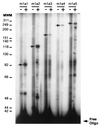
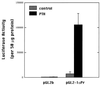
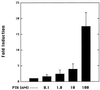
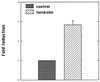
The DNA flanking the 5' sequence of the mouse 1alpha-hydroxylase gene has been cloned and sequenced. A TATA box has been located at -30 bp and aCCAAT box has been located at -79 bp. The gene's promoter activity has been demonstrated by using a luciferase reporter gene construct transfected into a modified pig kidney cell line, AOK-B50. Parathyroid hormone stimulates this promoter-directed synthesis of luciferase by 17-fold, whereas forskolin stimulates it by 3-fold. The action of parathyroid hormone is concentration-dependent. 1,25-Dihydroxyvitamin D3 does not suppress basal promoter activity and marginally suppresses parathyroid hormone-driven luciferase reporter activity. The promoter has three potential cAMP-responsive element sites, and two perfect and one imperfect AP-1 sites, while no DR-3 was detected. These results indicate that parathyroid hormone stimulates 25-hydroxyvitamin D3-1alpha-hydroxylase by acting on the promoter of the 1alpha-hydroxylase gene.
Figures


References
-
- DeLuca H F. The Harvey Lectures. New York: Academic; 1981. , Series 75, pp. 333–379.
-
- Ross T K, Darwish H M, DeLuca H F. Vitam Horm. 1994;49:281–326. - PubMed
-
- Prader A, Illig R, Heierli E. Helv Paediatr Acta. 1961;16:452–468. - PubMed
-
- Scriver C R. N Engl J Med. 1978;299:976–979. - PubMed
-
- Fraser D, Kooh S W, Kind H P, Holick M F, Tanaka Y, DeLuca H F. N Engl J Med. 1973;289:817–822. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

