RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1
- PMID: 9465032
- PMCID: PMC19032
- DOI: 10.1073/pnas.95.4.1432
RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1
Abstract
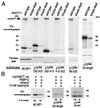
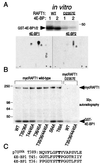
The complex of rapamycin with its intracellular receptor, FKBP12, interacts with RAFT1/FRAP/mTOR, the in vivo rapamycin-sensitive target and a member of the ataxia telangiectasia mutated (ATM)-related family of kinases that share homology with the catalytic domain of phosphatidylinositol 3-kinase. The function of RAFT1 in the rapamycin-sensitive pathway and its connection to downstream components of the pathway, such as p70 S6 kinase and 4E-BP1, are poorly understood. Here, we show that RAFT1 directly phosphorylates p70(S6k), 4E-BP1, and 4E-BP2 and that serum stimulates RAFT1 kinase activity with kinetics similar to those of p70(S6k) and 4E-BP1 phosphorylation. RAFT1 phosphorylates p70(S6k) on Thr-389, a residue whose phosphorylation is rapamycin-sensitive in vivo and necessary for S6 kinase activity. RAFT1 phosphorylation of 4E-BP1 on Thr-36 and Thr-45 blocks its association with the cap-binding protein, eIF-4E, in vitro, and phosphorylation of Thr-45 seems to be the major regulator of the 4E-BP1-eIF-4E interaction in vivo. RAFT1 phosphorylates p70(S6k) much more effectively than 4E-BP1, and the phosphorylation sites on the two proteins show little homology. This raises the possibility that, in vivo, an unidentified kinase analogous to p70(S6k) is activated by RAFT1 phosphorylation and acts at the rapamycin-sensitive phosphorylation sites of 4E-BP1.
Figures
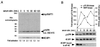


References
-
- Nielsen F C, Ostergaard L, Nielsen J, Christiansen J. Nature (London) 1995;377:358–362. - PubMed
-
- Brown E J, Schreiber S L. Cell. 1996;86:517–520. - PubMed
-
- Sonenberg N. In: Translational Control. Hershey J W B, Mathews M B, Sonenberg N, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 245–269.
-
- Martel R R, Klicius J, Galet S. Can J Physiol Pharmacol. 1977;55:48–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

