Is the molten globule a third phase of proteins?
- PMID: 9465042
- PMCID: PMC19058
- DOI: 10.1073/pnas.95.4.1490
Is the molten globule a third phase of proteins?
Abstract
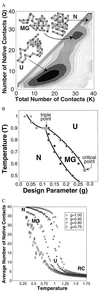
The equilibrium properties of proteins are studied by Monte Carlo simulation of two simplified models of protein-like heteropolymers. These models emphasize the polymeric entropy of the fluctuating polypeptide chain. Our calculations suggest a generic phase diagram that contains a thermodynamically distinct "molten globule" state in addition to a rigid native state and a nontrivial unfolded state. The roles of side-chain packing and loop entropy are discussed.
Figures


References
-
- Privalov P L. Adv Prot Chem. 1979;33:167–241. - PubMed
-
- Creighton T E. Protein Folding. New York: Freeman; 1992.
-
- Ptitsyn O B. Advances in Protein Chemistry. 1995;47:83–229. - PubMed
-
- Ptitsyn O B, Uversky V N. FEBS Lett. 1994;341:15–18. - PubMed
-
- Kuwajima K. Prot Struct Funct Genet. 1989;6:87–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

