Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence
- PMID: 9465043
- PMCID: PMC19060
- DOI: 10.1073/pnas.95.4.1495
Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence
Abstract
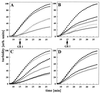
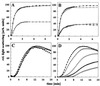
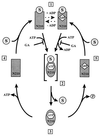
The abundant molecular chaperone Hsp90 is a key regulator of protein structure in the cytosol of eukaryotic cells. Although under physiological conditions a specific subset of proteins is substrate for Hsp90, under stress conditions Hsp90 seems to perform more general functions. However, the underlying mechanism of Hsp90 remained enigmatic. Here, we analyzed the function of conserved Hsp90 domains. We show that Hsp90 possesses two chaperone sites located in the N- and C-terminal fragments, respectively. The C-terminal fragment binds to partially folded proteins in an ATP-independent way potentially regulated by cochaperones. The N-terminal domain contains a peptide binding site that seems to bind preferentially peptides longer than 10 amino acids. Peptide dissociation is induced by ATP binding. Furthermore, the antitumor drug geldanamycin both inhibits the weak ATPase of Hsp90 and stimulates peptide release. We propose that the existence of two functionally different chaperone sites together with a substrate-selecting set of cochaperones allows Hsp90 to guide the folding of a subset of target proteins and, at the same time, to exhibit general chaperone functions.
Figures


References
-
- Buchner J. FASEB J. 1996;10:10–19. - PubMed
-
- Smith D F. Sci Med. 1995;2:38–47.
-
- Pratt W B, Toft D O. Endocr Rev. 1997;18:306–360. - PubMed
-
- Bohen S P, Yamamoto K R. In: The Biology of Heat Shock Proteins and Molecular Chaperones. Morimoto R, Tissières A, Georgopoulos C, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 313–334.
-
- Brugge J S. Curr Microbiol Immunol. 1986;123:1–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

