Monitoring conformational dynamics of a single molecule by selective fluorescence spectroscopy
- PMID: 9465054
- PMCID: PMC19090
- DOI: 10.1073/pnas.95.4.1556
Monitoring conformational dynamics of a single molecule by selective fluorescence spectroscopy
Abstract
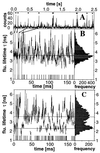
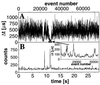
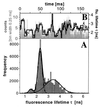
A recently developed, real-time spectroscopic technique, burst-integrated fluorescence lifetime (BIFL), is shown to be well suited for monitoring the individual molecular conformational dynamics of a single molecule diffusing through the microscopic, open measurement volume (approximately 10 fl) of a confocal epi-illuminated set-up. In a highly diluted aqueous solution of 20-mer oligonucleotide strand of DNA duplex labeled with the environment-sensitive fluorescent dye tetramethylrhodamine (TMR), fluorescence bursts indicating traces of individual molecules are registered and further subjected to selective burst analysis. The two-dimensional BIFL data allow the identification and detection of different temporally resolved conformational states. A complementary autocorrelation analysis was performed on the time-dependent fluctuations in fluorescence lifetime and intensity. The consistent results strongly support the hypothesized three-state model of the conformational dynamics of the TMR-DNA duplex with a polar, a nonpolar, and a quenching environment of TMR.
Figures





References
-
- Nie S, Chiu D T, Zare R N. Anal Chem. 1995;67:2849–2857.
-
- Keller R A, Ambrose W P, Goodwin P M, Jett J H, Martin J C, Ming W. Appl Spectrosc. 1996;50:12–32.
-
- Goodwin P M, Ambrose W P, Keller R A. Acc Chem Res. 1996;29:607–613.
-
- Affleck R L, Ambrose W P, Demas J N, Goodwin P M, Schecker J A, Wu M, Keller R A. Anal Chem. 1996;68:2270–2276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

