Molecular picture of folding of a small alpha/beta protein
- PMID: 9465055
- PMCID: PMC19093
- DOI: 10.1073/pnas.95.4.1562
Molecular picture of folding of a small alpha/beta protein
Abstract
We characterize, at the atomic level, the mechanism and thermodynamics of folding of a small alpha/beta protein. The thermodynamically significant states of segment B1 of streptococcal protein G (GB1) are probed by using the statistical mechanical methods of importance sampling and molecular dynamics. From a thermodynamic standpoint, folding commences with overall collapse, accompanied by formation of approximately 35% of the native structure. Specific contacts form at the loci experimentally inferred to be structured early in folding kinetics studies. Our study reveals that these initially structured regions are not spatially adjacent. As folding progresses, fluid-like nonlocal native contacts form, with many contacts forming and breaking as the structure searches for the native conformation. Although the alpha-helix forms early, the beta-sheet forms concomitantly with the overall topology. Water is present in the protein core up to a late stage of folding, lubricating conformational transitions during the search process. Once 80% of the native contacts have formed, water is squeezed from the protein interior and the structure descends into the native manifold. Examination of the onset of side-chain mobility within our model indicates side-chain motion is most closely linked to the overall volume of the protein and no sharp order-disorder transition appears to occur. Exploration of models for hydrogen deuterium exchange show qualitative agreement with equilibrium measurement of hydrogen/deuterium protection factors.
Figures
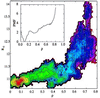


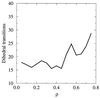

References
-
- Onuchic J N, Luthey-Schulten Z, Wolynes P G. Annu Rev Phys Chem. 1997;48:545–600. - PubMed
-
- Dill K A, Chan H S. Nat Struct Biol. 1997;4:10–19. - PubMed
-
- Abkevich V I, Gutin A M, Shakhnovich E I. J Chem Phys. 1994;101:6052–6062.
-
- Radford S E, Dobson C M. Phil Trans R Soc London B. 1995;348:17–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

