From differentiation to proliferation: the secretory amyloid precursor protein as a local mediator of growth in thyroid epithelial cells
- PMID: 9465092
- PMCID: PMC19185
- DOI: 10.1073/pnas.95.4.1770
From differentiation to proliferation: the secretory amyloid precursor protein as a local mediator of growth in thyroid epithelial cells
Abstract
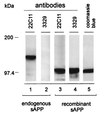
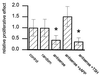
In various species, thyrotropin (TSH) is known to stimulate both differentiation and proliferation of thyroid follicle cells. This cell type has also been shown to express members of the Alzheimer amyloid precursor (APP) protein family and to release the secretory N-terminal domain of APP (sAPP) in a TSH-dependent fashion. In this study on binding to the cell surfaces, exogenously added recombinant sAPP stimulated phosphorylation mediated by mitogen-activated protein kinase and effectively evoked proliferation in the rat thyroid epithelial cell line FRTL-5. To see whether this proliverative effect of sAPP is of physiological relevance, we used antisense techniques to selectively inhibit the expression of APP and the proteolytic release of sAPP by cells grown in the presence of TSH. The antisense-induced inhibition was detected by immunoblot, immunoprecipitation, and immunocytochemical analyses. After the reduced APP expression and sAPP secretion, we observed a strong suppression of the TSH-induced cell proliferation down to 35%. Recombinant sAPP but not TSH was able to overcome this antisense effect and to completely restore cell proliferation, indicating that sAPP acts downstream of TSH, in that it is released from thyroid epithelial cells during TSH-induced differentiation. We propose that sAPP operates as an autocrine growth factor mediating the proliferative effect of TSH on neighboring thyroid epithelial cells.
Figures



References
-
- Sisodia S S, Price D L. FASEB J. 1995;9:366–370. - PubMed
-
- Selkoe D J. Annu Rev Cell Biol. 1994;10:373–403. - PubMed
-
- Sandbrink R, Masters C L, Beyreuther K. J Biol Chem. 1994;269:14227–14234. - PubMed
-
- Saitoh T, Roch J M, Jin L W, Ninomiya H, Otero D A C, Yamamoto K, Masliah E. In: Amyloid Precursor Protein in Development, Aging and Alzheimer’s Disease. Masters C L, Beyreuther K, Trillet M, Christen Y, editors. Heidelberg: Springer; 1994. pp. 90–99.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

