Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter
- PMID: 9465116
- PMCID: PMC19212
- DOI: 10.1073/pnas.95.4.1909
Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter
Abstract
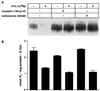
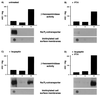
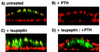
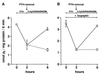
We have studied the involvement of proteolytic pathways in the regulation of the Na/Pi cotransporter type II by parathyroid hormone (PTH) in opossum kidney cells. Inhibition of lysosomal degradation (by leupeptin, ammonium chloride, methylamine, chloroquine, L-methionine methyl ester) prevented the PTH-mediated degradation of the transporter, whereas inhibition of the proteasomal pathway (by lactacystin) did not. Moreover it was found (i) that whereas lysosomal inhibitors prevented the PTH-mediated degradation of the transporter they did not prevent the PTH-mediated inhibition of the Na/Pi cotransport and (ii) that treating opossum kidney cells with lysosomal inhibitors led to an increased expression of the transporter without any concomitant increase in the Na/Pi cotransport. Further analysis by subcellular fractionation and morphological techniques showed (i) that the Na/Pi cotransporter is constitutively transported to and degraded within late endosomes/lysosomes and (ii) that PTH leads to the increased degradation of the transporter in late endosomes/lysosomes.
Figures

References
-
- Bradbury N A, Bridges R J. Am J Physiol. 1994;267:C1–C24. - PubMed
-
- James D E, Strube M, Mueckler M. Nature (London) 1989;338:83–87. - PubMed
-
- James D E, Brown R, Navarro J, Pilch P F. Nature (London) 1988;333:183–185. - PubMed
-
- Knepper M A, Wade J B, Terris J, Ecelbarger C A, Marples D, Mandon B, Chou C C, Kishore B K, Nilsen S. Kidney Int. 1996;49:1712–1717. - PubMed
-
- Kempson S A, Lötscher M, Kaissling B, Biber J, Murer H, Levi M. Am J Physiol. 1995;268:F784–F791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

