Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay
- PMID: 9466742
- PMCID: PMC104543
- DOI: 10.1128/JCM.36.2.362-366.1998
Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay
Abstract
A colorimetric, microplate-based Alamar Blue assay (MABA) method was used to determine the MICs of isoniazid (INH), rifampin, streptomycin (SM), and ethambutol (EMB) for 34 Peruvian Mycobacterium tuberculosis isolates (including both pansensitive and multidrug-resistant strains) and the H37Rv strain by using bacterial suspensions prepared directly from solid media. Results for all isolates were available within 8 days. Discordant results were observed on initial tests for 3 of 16 INH-susceptible isolates, 5 of 31 EMB-susceptible isolates, and 2 of 4 SM-resistant isolates (by the BACTEC 460 system). The overall agreements between the MICs obtained by MABA and the results obtained with the BACTEC 460 system were 87.9% for initial results and 93.6% after retesting 12 of 17 samples with discrepant results. Interpretation of MABA endpoints improved with technical experience. The MABA is a simple, rapid, low-cost, appropriate technology which does not require expensive instrumentation and which makes use of a nontoxic, temperature-stable reagent.
Figures
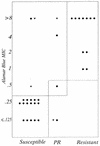


 isolates for
which results were discordant and for which MICs by MABA were not
redetermined; ▾, isolates for which results were discordant but for
which results were concordant upon repeat testing by MABA. The vertical
lines in the figures separate the standard breakpoints for the BACTEC
460 system. PR, partially resistant. The horizontal lines separate the
interpretive breakpoints for colorimetric MICs, which were selected on
the basis of the best fit of the MABA results with the BACTEC 460
system results.
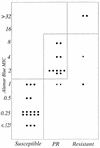
isolates for
which results were discordant and for which MICs by MABA were not
redetermined; ▾, isolates for which results were discordant but for
which results were concordant upon repeat testing by MABA. The vertical
lines in the figures separate the standard breakpoints for the BACTEC
460 system. PR, partially resistant. The horizontal lines separate the
interpretive breakpoints for colorimetric MICs, which were selected on
the basis of the best fit of the MABA results with the BACTEC 460
system results.References
-
- Cohn, D. L., F. Bustreo, and M. C. Raviglione. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD global surveillance project. Clin. Infect. Dis. 24(Suppl. 1):S121–S130. - PubMed
-
- Collins, L., and S. Franzblau. Unpublished data.
-
- Franzblau S G, Witzig R S, Gilman R H, Madico G, McLaughlin J, Cook M B, Torres P, Fuentes P, McGuire M S. Abstracts of the 45th Annual Meeting of the American Society of Tropical Medicine and Hygiene. 1996. Rapid drug susceptibility assay for developing countries using Alamar Blue, abstr. 335; p. 210.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

