Cdc7 is required throughout the yeast S phase to activate replication origins
- PMID: 9472018
- PMCID: PMC316537
- DOI: 10.1101/gad.12.4.491
Cdc7 is required throughout the yeast S phase to activate replication origins
Abstract
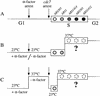
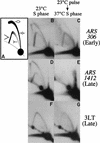
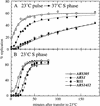
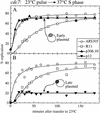
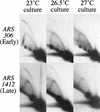
The long-standing conclusion that the Cdc7 kinase of Saccharomyces cerevisiae is required only to trigger S phase has been challenged by recent data that suggests it acts directly on individual replication origins. We tested the possibility that early- and late-activated origins have different requirements for Cdc7 activity. Cells carrying a cdc7(ts) allele were first arrested in G1 at the cdc7 block by incubation at 37 degrees C, and then were allowed to enter S phase by brief incubation at 23 degrees C. During the S phase, after return to 37 degrees C, early-firing replication origins were activated, but late origins failed to fire. Similarly, a plasmid with a late-activated origin was defective in replication. As a consequence of the origin activation defect, duplication of chromosomal sequences that are normally replicated from late origins was greatly delayed. Early-replicating regions of the genome duplicated at approximately their normal time. The requirements of early and late origins for Cdc7 appear to be temporally rather than quantitatively different, as reducing overall levels of Cdc7 by growth at semi-permissive temperature reduced activation at early and late origins approximately equally. Our results show that Cdc7 activates early and late origins separately, with late origins requiring the activity later in S phase to permit replication initiation.
Figures

References
-
- Bahman M, Buck V, White A, Rosamond J. Characterisation of the CDC7 gene product of Saccharomyces cerevisiae as a protein kinase needed for the initiation of mitotic DNA synthesis. Biochim Biophys Acta. 1988;951:335–343. - PubMed
-
- Brewer BJ, Fangman WL. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987;51:463–471. - PubMed
-
- ————— Mapping replication origins in yeast chromosomes. BioEssays. 1991;13:317–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
