The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase
- PMID: 9472021
- PMCID: PMC316522
- DOI: 10.1101/gad.12.4.527
The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase
Abstract
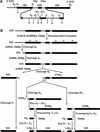
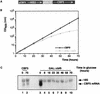
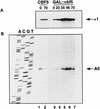
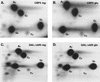
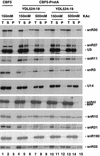
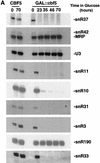
Many or all of the sites of pseudouridine (Psi) formation in eukaryotic rRNA are selected by site-specific base-pairing with members of the box H + ACA class of small nucleolar RNAs (snoRNAs). Database searches previously identified strong homology between the rat nucleolar protein Nap57p, its yeast homolog Cbf5p, and the Escherichia coli Psi synthase truB/P35. We therefore tested whether Cbf5p is required for synthesis of Psi in the yeast rRNA. After genetic depletion of Cbf5p, formation of Psi in the pre-rRNA is dramatically inhibited, resulting in accumulation of the unmodified rRNA. Protein A-tagged Cbf5p coprecipitates all tested members of the box H + ACA snoRNAs but not box C + D snoRNAs or other RNA species. Genetic depletion of Cbf5p leads to depletion of all box H + ACA snoRNAs. These include snR30, which is required for pre-rRNA processing. Depletion of Cbf5p also results in a pre-rRNA processing defect similar to that seen on depletion of snR30. We conclude that Cbf5p is likely to be the rRNA Psi synthase and is an integral component of the box H + ACA class of snoRNPs, which function to target the enzyme to its site of action.
Figures






References
-
- Abou Elela S, Igel H, Ares M., Jr RNase III cleaves eukaryotic preribosomal RNA at a U3 snoRNP-dependent site. Cell. 1996;85:115–124. - PubMed
-
- Bachellerie JP, Cavaillé J. Guiding ribose methylation of rRNA. Trends Biochem Sci. 1997;22:257–261. - PubMed
-
- Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: Two major families of small RNAs defined by different box elements with related functions. Cell. 1996;85:823–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
