Different core promoters possess distinct regulatory activities in the Drosophila embryo
- PMID: 9472023
- PMCID: PMC316525
- DOI: 10.1101/gad.12.4.547
Different core promoters possess distinct regulatory activities in the Drosophila embryo
Abstract
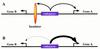
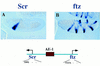
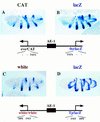
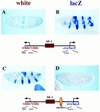
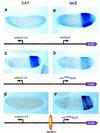
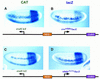
There are numerous examples of shared enhancers interacting with just a subset of target promoters. In some cases, specific enhancer-promoter interactions depend on promoter competition, whereby the activation of a preferred target promoter precludes expression of linked genes. Here, we employ a transgenic embryo assay to obtain evidence that promoter selection is influenced by the TATA element. Both the AE1 enhancer from the Drosophila Antennapedia gene complex (ANT-C) and the IAB5 enhancer from the Bithorax complex (BX-C) preferentially activate TATA-containing promoters when challenged with linked TATA-less promoters. In contrast, the rho neuroectoderm enhancer (NEE) does not discriminate between these two classes of promoters. Thus, certain upstream activators, such as Ftz, prefer TATA-containing promoters, whereas other activators, including Dorsal, work equally well on both classes of promoters. These results provide in vivo evidence that different core promoters possess distinct regulatory activities. We discuss the possibility that an invariant TFIID complex can adopt different conformations on the core promoter.
Figures



References
-
- Arnosti DN, Barolo S, Levine M, Small S. The eve stripe 2 enhancer employs multiple modes of transcriptional synergy. Development. 1996;122:205–214. - PubMed
-
- Burke TW, Kadonaga JT. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes & Dev. 1996;10:711–724. - PubMed
-
- Burley SK, Roeder RG. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
