gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer
- PMID: 9472029
- PMCID: PMC2141742
- DOI: 10.1083/jcb.140.4.751
gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer
Abstract
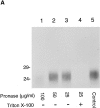
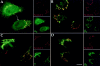
Abstract. Five mammalian members of the gp25L/ emp24/p24 family have been identified as major constituents of the cis-Golgi network of rat liver and HeLa cells. Two of these were also found in membranes of higher density (corresponding to the ER), and this correlated with their ability to bind COP I in vitro. This binding was mediated by a K(X)KXX-like retrieval motif present in the cytoplasmic domain of these two members. A second motif, double phenylalanine (FF), present in the cytoplasmic domain of all five members, was shown to participate in the binding of Sec23 (COP II). This motif is part of a larger one, similar to the F/YXXXXF/Y strong endocytosis and putative AP2 binding motif. In vivo mutational analysis confirmed the roles of both motifs so that when COP I binding was expected to be impaired, cell surface expression was observed, whereas mutation of the Sec23 binding motif resulted in a redistribution to the ER. Surprisingly, upon expression of mutated members, steady-state distribution of unmutated ones shifted as well, presumably as a consequence of their observed oligomeric properties.
Figures








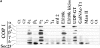

References
-
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;6:315–320. - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

