The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death
- PMID: 9472042
- PMCID: PMC2141754
- DOI: 10.1083/jcb.140.4.911
The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death
Abstract
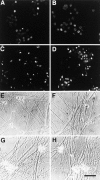
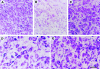
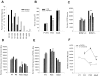
To determine whether the p75 neurotrophin receptor (p75NTR) plays a role in naturally occurring neuronal death, we examined neonatal sympathetic neurons that express both the TrkA tyrosine kinase receptor and p75NTR. When sympathetic neuron survival is maintained with low quantities of NGF or KCl, the neurotrophin brain-derived neurotrophic factor (BDNF), which does not activate Trk receptors on sympathetic neurons, causes neuronal apoptosis and increased phosphorylation of c-jun. Function-blocking antibody studies indicate that this apoptosis is due to BDNF-mediated activation of p75NTR. To determine the physiological relevance of these culture findings, we examined sympathetic neurons in BDNF-/- and p75NTR-/- mice. In BDNF-/- mice, sympathetic neuron number is increased relative to BDNF+/+ littermates, and in p75NTR-/- mice, the normal period of sympathetic neuron death does not occur, with neuronal attrition occurring later in life. This deficit in apoptosis is intrinsic to sympathetic neurons, since cultured p75NTR-/- neurons die more slowly than do their wild-type counterparts. Together, these data indicate that p75NTR can signal to mediate apoptosis, and that this mechanism is essential for naturally occurring sympathetic neuron death.
Figures


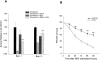
References
-
- Barbacid M. The trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
-
- Barde Y-A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. - PubMed
-
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

