Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology
- PMID: 9480986
- PMCID: PMC2212172
- DOI: 10.1084/jem.187.5.763
Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology
Abstract
We studied the impact of various infectious and proinflammatory agents on the induction of peripheral T cell tolerance. Adoptive transfer of CD8+ T cells from lymphocytic choriomeningitis virus (LCMV) T cell receptor transgenic mice into LCMV antigen transgenic mice expressing the LCMV glycoprotein epitope (gp) 33-41 under control of a major histocompatibility complex class I promoter led to efficient induction of peripheral tolerance after a period of transient activation. If, however, the recipient mice were challenged with viral or bacterial infections or proinflammatory agents (lipopolysaccharide or Poly:IC) early after cell transfer, tolerance induction was prevented and instead, CD8+ T cell activation leading to vigorous expansion and generation of cytolytic activity ensued. This became manifest in significant immunopathology mainly involving destruction of the splenic architecture and lysis of antigen-expressing lymphocyte and macrophage populations. Important parameters involved in the activation of host-reactive T cells by nonspecific infectious agents included the presence, localization, and quantity of the specific transgene-encoded self-antigen; in contrast, CD4+ T cells were not required. In mice surviving the acute phase, the transferred CD8+ T cells persisted at high levels in an anergic state; they were unable to generate cytolytic activity in vitro or to control LCMV infection in vivo. These results impinge on our understanding of the role of infectious agents in graft verus host reactions towards minor histocompatibility antigens.
Figures


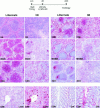
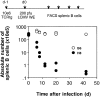




References
-
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. - PubMed
-
- Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. - PubMed
-
- Zinkernagel R, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localization regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. - PubMed
-
- Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt VA, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. - PubMed
-
- Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

