Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells
- PMID: 9480988
- PMCID: PMC2212173
- DOI: 10.1084/jem.187.5.787
Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells
Abstract
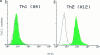
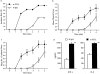
T helper cell type 1 (Th1) and 2 (Th2) are central to immune regulation. However, no stable cell surface marker capable of distinguishing and separating these two subsets of CD4(+) cells has yet been found. Using differential display PCR, we have identified a gene encoding a cell membrane bound molecule, originally designated ST2L, T1, DER4, or Fit, expressed constitutively and stably on the surface of murine Th2s, but not Th1s even after stimulation with a range of immunological stimuli. Antibody against a peptide derived from ST2L strongly and stably labeled the surface of cloned Th2s but not Th1s, and Th2s but not Th1s derived from naive T cells of ovalbumin T cell receptor-alpha/beta transgenic mice. Three-color single cell flow cytometric analysis shows that cell surface ST2L coexpressed with intracellular interleukin (IL)-4, but not with interferon (IFN)-gamma. The antibody selectively lysed Th2s in vitro in a complement-dependent manner. In vivo, it enhanced Th1 responses by increasing IFN-gamma production and decreasing IL-4 and IL-5 synthesis. It induced resistance to Leishmania major infection in BALB/c mice and exacerbated collagen-induced arthritis in DBA/1 mice. Thus, ST2L is a stable marker distinguishing Th2s from Th1s and is also associated with Th2 functions. Hence, it may be a target for therapeutic intervention.
Figures







References
-
- Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell–derived cytokines. Annu Rev Immunol. 1992;10:385–409. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Delprete G, Defcarli M, Almerigogna F, Daniel CK, Delios MM, Zancuoghi G, Vinante F, Pizzolo G, Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

