Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem
- PMID: 9481678
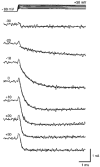
- PMCID: PMC2230710
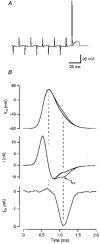
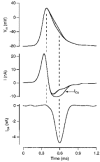
- DOI: 10.1111/j.1469-7793.1998.143bx.x
Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem
Abstract
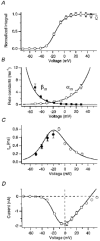
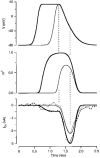
1. The calcium current of a 'giant' synaptic terminal (the calyx of Held) was studied using two-electrode voltage clamp in slices of the rat brainstem. 2. In terminals with a long axon (length > 100 microns), the passive current transient decayed biexponentially following voltage steps. In terminals with a short axon (length < 30 microns), the slow component was reduced or absent. These terminals also had small slow calcium tail currents following long depolarizing voltage steps, suggesting that these are largely due to axonal calcium channels. 3. Terminals were voltage clamped with action potential waveform commands. At both 24 and 36 degrees C the calcium current began shortly after the peak of the action potential and ended before the terminal was fully repolarized. 4. The calcium current during the repolarization phase was 69 +/- 1% (n = 3) of maximal, judged from the increase in this current when a plateau phase was added to the action potential waveform. 5. A Hodgkin-Huxley m2 model, based on the measured activation and deactivation of the calcium current, reproduced both the time course and the amplitude increase of the calcium currents during the different action potential waveforms well. 6. The fast gating of the calcium channels in the terminal ensures that they are effectively opened during the repolarization phase of an action potential. This implies that the distance between open calcium channels is minimized, which is in agreement with the view that multiple calcium channels are needed to release a vesicle in this synapse.
Figures



References
-
- Bertram R, Sherman A, Stanley EF. Single-domain/bound calcium hypothesis of transmitter release and facilitation. Journal of Neurophysiology. 1996;75:1919–1931. - PubMed
-
- Borst JGG, Helmchen F. Calcium influx during an action potential. In: Conn PM, editor. Methods in Enzymology. Academic Press; 1998. in the Press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

