Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons
- PMID: 9482781
- PMCID: PMC6792914
- DOI: 10.1523/JNEUROSCI.18-06-01953.1998
Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons
Abstract
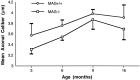
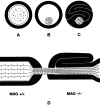
Myelination increases neuronal conduction velocity through its insulating properties and an unidentified extrinsic effect that increases axonal caliber. Although it is well established that demyelination can cause axonal atrophy, the myelin molecule that regulates axonal caliber is not known. Loss of the structural proteins of compact peripheral nervous system (PNS) myelin, P0 protein, and myelin basic protein does not lead to axonal atrophy. This study demonstrates that mice with a null mutation of the myelin-associated glycoprotein (MAG) gene have a chronic atrophy of myelinated PNS axons that results in paranodal myelin tomaculi and axonal degeneration. Absence of MAG was correlated with reduced axonal calibers, decreased neurofilament spacing, and reduced neurofilament phosphorylation. Because axonal atrophy and degeneration in MAG-deficient mice occur in the absence of inflammation, hypomyelination, significant demyelination-remyelination, or gain of function mutations, these data support a functional role for MAG in modulating the maturation and viability of myelinated axons.
Figures




References
-
- Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. - PubMed
-
- Carenini S, Montag D, Cremer H, Schachner M, Martini R. Absence of the myelin-associated glycoprotein (MAG) and the neural cell adhesion molecule (N-CAM) interferes with the maintenance, but not with the formation of peripheral myelin. Cell Tissue Res. 1997;287:3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
