Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo
- PMID: 9482801
- PMCID: PMC6792921
- DOI: 10.1523/JNEUROSCI.18-06-02161.1998
Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo
Abstract
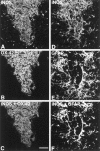
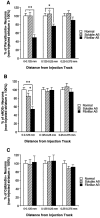
To determine the stability of beta-amyloid peptide (Abeta) and the glial and neuronal changes induced by Abeta in the CNS in vivo, we made single injections of fibrillar Abeta (fAbeta), soluble Abeta (sAbeta), or vehicle into the rat striatum. Injected fAbeta is stable in vivo for at least 30 d after injection, whereas sAbeta is primarily cleared within 1 d. After injection of fAbeta, microglia phagocytize fAbeta aggregates, whereas nearby astrocytes form a virtual wall between fAbeta-containing microglia and the surrounding neuropil. Similar glial changes are not observed after sAbeta injection. Microglia and astrocytes near the injected fAbeta show a significant increase in inducible nitric oxide synthase (iNOS) expression compared with that seen with sAbeta or vehicle injection. Injection of fAbeta but not sAbeta or vehicle induces a significant loss of parvalbumin- and neuronal nitric oxide synthase-immunoreactive neurons, whereas the number of calbindin-immunoreactive neurons remains unchanged. These data demonstrate that fAbeta is remarkably stable in the CNS in vivo and suggest that fAbeta neurotoxicity is mediated in large part by factors released from activated microglia and astrocytes, as opposed to direct interaction between Abeta fibrils and neurons.
Figures






References
-
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase–elevation in severe AIDS dementia and induction by HIV-1 Gp41. Science. 1996;274:1917–1921. - PubMed
-
- Akiyama H, Schwab C, Kondo H, Mori H, Kametani F, Ikeda K, McGeer PL. Granules in glial cells of patients with Alzheimer’s disease are immunopositive for C-terminal sequences of beta-amyloid protein. Neurosci Lett. 1996;206:169–172. - PubMed
-
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer’s amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. - PubMed
-
- Barron KD. The microglial cell. A historical review. J Neurol Sci. 1995;134:57–68. - PubMed
-
- Bennett BD, Bolam JP. Two populations of calbindin D28k-immunoreactive neurones in the striatum of the rat. Brain Res. 1993;610:305–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
