Refractoriness and neural precision
- PMID: 9482804
- PMCID: PMC6792934
- DOI: 10.1523/JNEUROSCI.18-06-02200.1998
Refractoriness and neural precision
Abstract
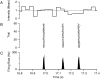
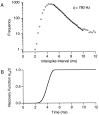
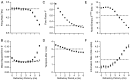
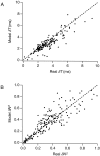
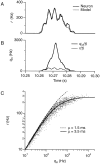
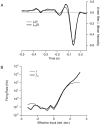
The response of a spiking neuron to a stimulus is often characterized by its time-varying firing rate, estimated from a histogram of spike times. If the cell's firing probability in each small time interval depends only on this firing rate, one predicts a highly variable response to repeated trials, whereas many neurons show much greater fidelity. Furthermore, the neuronal membrane is refractory immediately after a spike, so that the firing probability depends not only on the stimulus but also on the preceding spike train. To connect these observations, we investigated the relationship between the refractory period of a neuron and its firing precision. The light response of retinal ganglion cells was modeled as probabilistic firing combined with a refractory period: the instantaneous firing rate is the product of a "free firing rate, " which depends only on the stimulus, and a "recovery function," which depends only on the time since the last spike. This recovery function vanishes for an absolute refractory period and then gradually increases to unity. In simulations, longer refractory periods were found to make the response more reproducible, eventually matching the precision of measured spike trains. Refractoriness, although often thought to limit the performance of neurons, may in fact benefit neuronal reliability. The underlying free firing rate derived by allowing for the refractory period often exceeded the observed firing rate by an order of magnitude and was found to convey information about the stimulus over a much wider dynamic range. Thus, the free firing rate may be the preferred variable for describing the response of a spiking neuron.
Figures



References
-
- Abeles M. Corticonics: neural circuits of the cerebral cortex. Cambridge UP; Cambridge: 1991.
-
- Bi Q. A closed-form solution for removing the dead time effects from the poststimulus time histograms. J Acoust Soc Am. 1989;85:2504–2513. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
