Feature extraction by burst-like spike patterns in multiple sensory maps
- PMID: 9482813
- PMCID: PMC6792916
- DOI: 10.1523/JNEUROSCI.18-06-02283.1998
Feature extraction by burst-like spike patterns in multiple sensory maps
Abstract
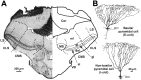
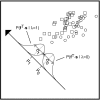
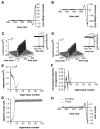
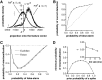
In most sensory systems, higher order central neurons extract those stimulus features from the sensory periphery that are behaviorally relevant (e.g.,Marr, 1982; Heiligenberg, 1991). Recent studies have quantified the time-varying information carried by spike trains of sensory neurons in various systems using stimulus estimation methods (Bialek et al., 1991; Wessel et al., 1996). Here, we address the question of how this information is transferred from the sensory neuron level to higher order neurons across multiple sensory maps by using the electrosensory system in weakly electric fish as a model. To determine how electric field amplitude modulations are temporally encoded and processed at two subsequent stages of the amplitude coding pathway, we recorded the responses of P-type afferents and E- and I-type pyramidal cells in the electrosensory lateral line lobe (ELL) to random distortions of a mimic of the fish's own electric field. Cells in two of the three somatotopically organized ELL maps were studied (centromedial and lateral) (Maler, 1979; Carr and Maler, 1986). Linear and second order nonlinear stimulus estimation methods indicated that in contrast to P-receptor afferents, pyramidal cells did not reliably encode time-varying information about any function of the stimulus obtained by linear filtering and half-wave rectification. Two pattern classifiers were applied to discriminate stimulus waveforms preceding the occurrence or nonoccurrence of pyramidal cell spikes in response to the stimulus. These signal-detection methods revealed that pyramidal cells reliably encoded the presence of upstrokes and downstrokes in random amplitude modulations by short bursts of spikes. Furthermore, among the different cell types in the ELL, I-type pyramidal cells in the centromedial map performed a better pattern-recognition task than those in the lateral map and than E-type pyramidal cells in either map.
Figures

Similar articles
-
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning.J Physiol Paris. 2008 Jul-Nov;102(4-6):195-208. doi: 10.1016/j.jphysparis.2008.10.019. Epub 2008 Oct 18. J Physiol Paris. 2008. PMID: 18992813 Free PMC article. Review.
-
From stimulus encoding to feature extraction in weakly electric fish.Nature. 1996 Dec 12;384(6609):564-7. doi: 10.1038/384564a0. Nature. 1996. PMID: 8955269
-
Stimulus encoding and feature extraction by multiple sensory neurons.J Neurosci. 2002 Mar 15;22(6):2374-82. doi: 10.1523/JNEUROSCI.22-06-02374.2002. J Neurosci. 2002. PMID: 11896176 Free PMC article.
-
Oscillatory and burst discharge across electrosensory topographic maps.J Neurophysiol. 1996 Oct;76(4):2364-82. doi: 10.1152/jn.1996.76.4.2364. J Neurophysiol. 1996. PMID: 8899610
-
Perception and coding of envelopes in weakly electric fishes.J Exp Biol. 2013 Jul 1;216(Pt 13):2393-402. doi: 10.1242/jeb.082321. J Exp Biol. 2013. PMID: 23761464 Free PMC article. Review.
Cited by
-
The contribution of dendritic Kv3 K+ channels to burst threshold in a sensory neuron.J Neurosci. 2001 Jan 1;21(1):125-35. doi: 10.1523/JNEUROSCI.21-01-00125.2001. J Neurosci. 2001. PMID: 11150328 Free PMC article.
-
Burst Firing in a Motion-Sensitive Neural Pathway Correlates with Expansion Properties of Looming Objects that Evoke Avoidance Behaviors.Front Integr Neurosci. 2015 Dec 14;9:60. doi: 10.3389/fnint.2015.00060. eCollection 2015. Front Integr Neurosci. 2015. PMID: 26696845 Free PMC article.
-
Feedback and feedforward control of frequency tuning to naturalistic stimuli.J Neurosci. 2005 Jun 8;25(23):5521-32. doi: 10.1523/JNEUROSCI.0445-05.2005. J Neurosci. 2005. PMID: 15944380 Free PMC article.
-
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning.J Physiol Paris. 2008 Jul-Nov;102(4-6):195-208. doi: 10.1016/j.jphysparis.2008.10.019. Epub 2008 Oct 18. J Physiol Paris. 2008. PMID: 18992813 Free PMC article. Review.
-
Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity.J Neurosci. 2002 Jun 1;22(11):4577-90. doi: 10.1523/JNEUROSCI.22-11-04577.2002. J Neurosci. 2002. PMID: 12040065 Free PMC article.
References
-
- Anderson TW. An introduction to multivariate statistical analysis, Ed 2. Wiley; New York: 1984.
-
- Anderson TW, Bahadur RR. Classification into two multivariate normal distributions with different covariance matrices. Ann Math Stat. 1962;33:420–431.
-
- Aziz PM, Sorensen HV, Van der Spiegel J. An overview of sigma-delta converters. IEEE Sig Proc Mag. 1996;13:61–84.
-
- Bastian J. Electrolocation: I. The effects of moving objects and other electric stimuli on the activities of two categories of posterior lateral line cells in Apteronotus albifrons. J Comp Physiol. 1981a;144:481–494.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
