Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina
- PMID: 9482814
- PMCID: PMC6792907
- DOI: 10.1523/JNEUROSCI.18-06-02301.1998
Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina
Abstract
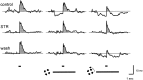
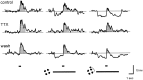
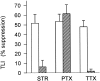
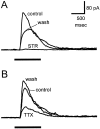
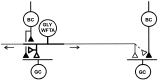
Transient lateral inhibition (TLI), the suppression of responses of a ganglion cell to light stimuli in the receptive field center by changes in illumination in the receptive field surround, was studied in light-adapted mud puppy and tiger salamander retinas using both eyecup and retinal slice preparations. In the eyecup, TLI was measured in on-off ganglion cells as the ability of rotating, concentric windmill patterns of 500-1200 micron inner diameter to suppress the response to a small spot stimulus in the receptive field center. Both the suppression of the spot response and the hyperpolarization produced in ganglion cells by rotation of the windmill were blocked in the presence of 2 microM strychnine or 500 nM tetrodotoxin (TTX), but not by 150 microM picrotoxin. In the slice preparation in which GABA-mediated currents were blocked with picrotoxin, IPSCs elicited by diffuse illumination were blocked by strychnine and strongly reduced by TTX. The TTX-resistant component was probably attributable to illumination of the receptive field center. TTX had a much greater effect in reducing the glycinergic inhibition elicited by laterally displaced stimulation versus nearby focal electrical stimulation. Strychnine enhanced light-evoked excitatory currents in ganglion cells, but this was not mimicked by TTX. The results suggest that local glycinergic transient inhibition does not require action potentials and is mediated by synapses onto both ganglion cell dendrites and bipolar cell terminals. In contrast, the lateral spread of this inhibition (at least over distances >250 micron) requires action potentials and is mainly onto ganglion cell dendrites.
Figures

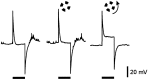



References
-
- Barnes S, Werblin FS. Direct excitatory and lateral inhibitory synaptic inputs to amacrine cells in the tiger salamander retina. Brain Res. 1987;406:233–237. - PubMed
-
- Bloomfield S. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol. 1996;75:1878–1893. - PubMed
-
- Cook PB, McReynolds JS. Modulation of sustained and transient lateral inhibitory mechanisms in the mud puppy retina during light adaptation. J Neurophysiol. 1998;79:197–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
