A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases
- PMID: 9482839
- PMCID: PMC19251
- DOI: 10.1073/pnas.95.5.2067
A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases
Abstract
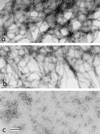
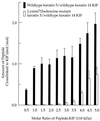
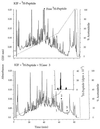
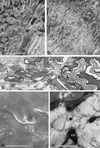
We have addressed the question of how keratin intermediate filaments are associated with the cell envelope at the periphery of cornified epidermal cells. Many peptides from human epidermal cell envelopes containing isopeptide crosslinks inserted by transglutaminases in vivo have been characterized. A major subset involves the type II keratin chains keratin 1, 2e, 5, or 6 crosslinked to several protein partners through a lysine residue located in a conserved region of the V1 subdomain of their head domains. This sequence specificity was confirmed in in vitro crosslinking experiments. Previously the causative mutation in a family with diffuse nonepidermolytic palmar-plantar keratoderma was shown to be the loss in one allele of the same lysine residue of the keratin 1 chain. Ultrastructural studies of affected palm epidermis have revealed abnormalities in the organization of keratin filaments subjacent to the cell envelope and in the shape of the cornified cells. Together, these data suggest a mechanism for the coordination of cornified cell structure by permanent covalent attachment of the keratin intermediate filament cytoskeleton to the cell envelope by transglutaminase crosslinking. Furthermore, these studies identify the essential role of a conserved lysine residue on the head domains of type II keratins in the supramolecular organization of keratin filaments in cells.
Figures

References
-
- Hohl D. Dermatologica. 1990;180:201–211. - PubMed
-
- Greenberg C S, Birckbichler P J, Rice R H. FASEB J. 1991;5:3071–3077. - PubMed
-
- Reichert U, Michel S, Schmidt R. In: Molecular Biology of the Skin. Darmon M, Blumenberg M, editors. New York: Academic; 1993. pp. 107–150.
-
- Holbrook K A, Wolff K. In: Dermatology in General Medicine. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. New York: McGraw–Hill; 1993. pp. 97–145.
-
- Elias P M. J Dermatol. 1996;23:756–758. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

