A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway
- PMID: 9482845
- PMCID: PMC19263
- DOI: 10.1073/pnas.95.5.2100
A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway
Abstract
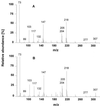
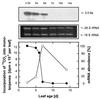
Isopentenyl diphosphate, the common precursor of all isoprenoids, has been widely assumed to be synthesized by the acetate/mevalonate pathway in all organisms. However, based on in vivo feeding experiments, isopentenyl diphosphate formation in several eubacteria, a green alga, and plant chloroplasts has been demonstrated very recently to originate via a mevalonate-independent route from pyruvate and glyceraldehyde 3-phosphate as precursors. Here we describe the cloning from peppermint (Mentha x piperita) and heterologous expression in Escherichia coli of 1-deoxy-D-xylulose-5-phosphate synthase, the enzyme that catalyzes the first reaction of this pyruvate/glyceraldehyde 3-phosphate pathway. This synthase gene contains an ORF of 2,172 base pairs. When the proposed plastid targeting sequence is excluded, the deduced amino acid sequence indicates the peppermint synthase to be about 650 residues in length, corresponding to a native size of roughly 71 kDa. The enzyme appears to represent a novel class of highly conserved transketolases and likely plays a key role in the biosynthesis of plastid-derived isoprenoids essential for growth, development, and defense in plants.
Figures


References
-
- Connolly J D, Hill R A. Dictionary of Terpenoids. London: Chapman & Hall; 1991.
-
- Langenheim J H. J Chem Ecol. 1994;20:1223–1280. - PubMed
-
- Spurgeon S L, Porter J W. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 1–46.
-
- Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

