Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation
- PMID: 9482883
- PMCID: PMC19331
- DOI: 10.1073/pnas.95.5.2319
Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation
Abstract
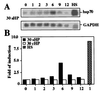
In response to various stress stimuli, heat shock genes are induced to express heat shock proteins (Hsps). Previous studies have revealed that expression of heat shock genes is regulated both at transcriptional and posttranscriptional level, and the rapid transcriptional induction of heat shock genes involves activation of the specific transcription factor, heat shock factor 1 (HSF1). Furthermore, the transcriptional induction can vary in intensity and kinetics in a signal- and cell-type-dependent manner. In this study, we demonstrate that mechanical loading in the form of hydrostatic pressure increases heat shock gene expression in human chondrocyte-like cells. The response to continuous high hydrostatic pressure was characterized by elevated mRNA and protein levels of Hsp70, without activation of HSF1 and transcriptional induction of hsp70 gene. The increased expression of Hsp70 was mediated through stabilization of hsp70 mRNA molecules. Interestingly, in contrast to static pressurization, cyclic hydrostatic loading did not result in the induction of heat shock genes. Our findings show that hsp70 gene expression is regulated posttranscriptionally without transcriptional induction in chondrocyte-like cells upon exposure to high continuous hydrostatic pressure. We suggest that the posttranscriptional regulation in the form of hsp70 mRNA stabilization provides an additional mode of heat shock gene regulation that is likely to be of significant importance in certain forms of stress.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

