Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition
- PMID: 9482917
- PMCID: PMC19396
- DOI: 10.1073/pnas.95.5.2515
Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition
Abstract
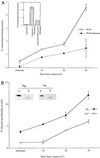
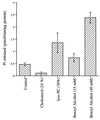
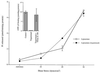
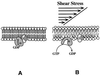
Mechanical forces arising from strain, pressure, and fluid shear stress are sensed by cells through an unidentified mechanoreceptor(s) coupled to intracellular signaling pathways. In vascular endothelial cells, fluid shear stress is transduced via pathway(s) involving heterotrimeric guanine nucleotide-binding proteins (G proteins) by molecular mechanisms that are unknown. In the present study, we investigated the activation of purified G proteins reconstituted into phospholipid vesicles. Vesicles containing G proteins were loaded with [gamma-32P]GTP and subjected to physiological levels of fluid shear stress in a cone-and-plate viscometer. Steady-state GTP hydrolysis was measured as an index of G protein function. Shear stress (0-30 dynes/cm2) activated G proteins in dose-dependent manner (0.48-4.6 pmol/min per microg of protein). Liposomes containing lysophosphatidylcholine (30 mol %) or treated with benzyl alcohol (40 mM), conditions that increase bilayer fluidity, exhibited 3- to 5-fold enhancement of basal GTPase activity. Conversely, incorporation of cholesterol (24 mol %) into liposomes reduced the activation of G proteins by shear. These results demonstrate the ability of the phospholipid bilayer to mediate the shear stress-induced activation of membrane-bound G proteins in the absence of protein receptors and that bilayer physical properties modulate this response.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

