Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells
- PMID: 9486003
- PMCID: PMC1752416
- DOI: 10.1136/ard.56.7.414
Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells
Abstract
Objective: In aseptic loosening, a heavy macrophage response to biomaterial wear particles is commonly found in arthroplasty tissues. The aim of this study was to discover if these cells contribute to the bone resorption of aseptic loosening by differentiating into osteoclasts.
Methods: Macrophages were isolated from the pseudocapsule and pseudomembrane of loose cemented and uncemented hip arthroplasties at the time of revision surgery and then co-cultured on glass coverslips and dentine slices with UMR 106 rat osteoblast-like cells, both in the presence and absence of 1,25 dihydroxyvitamin D3 [1,25(OH)2D3]. Macrophages isolated from the synovial membrane of patients with osteoarthritis (OA) undergoing hip replacements were similarly studied as a control group.
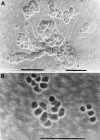
Results: After 24 hours incubation, most cells isolated from the above periprosthetic tissues strongly expressed macrophage (CD11b, CD14) but not osteoclast markers. However, after 14 days incubation, numerous multinucleated cells showing the phenotypic features of osteoclasts (that is, positive for tartrate resistant acid phosphatase, the vitronectin receptor, and capable of extensive lacunar resorption) formed in co-cultures of arthroplasty derived macrophages and UMR 106 cells, in the presence of 1,25(OH)2D3. The addition of an antibody to macrophage colony stimulating factor (M-CSF) considerably reduced macrophage-osteoclast differentiation and hence the lacunar resorption seen in these co-cultures. In contrast, OA synovial macrophage/UMR 106 co-cultures showed little or no evidence of macrophage-osteoclast differentiation and this was only seen when human M-CSF was added to the co-cultures.
Conclusion: This is the first report showing that human macrophages isolated directly from periprosthetic tissues surrounding loosened implants can differentiate into multinucleated cells showing all the functional and cytochemical characteristics of osteoclasts. In contrast with other macrophage populations, exogenous M-CSF is not required for this to occur. In the context of the heavy macrophage response to wear particles in periprosthetic tissues macrophage-osteoclast differentiation may represent an important cellular mechanism whereby osteolysis is effected in aseptic loosening.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

