HLA class II phenotype controls diversification of the autoantibody response in primary Sjögren's syndrome (pSS)
- PMID: 9486405
- PMCID: PMC1904917
- DOI: 10.1046/j.1365-2249.1998.00504.x
HLA class II phenotype controls diversification of the autoantibody response in primary Sjögren's syndrome (pSS)
Abstract
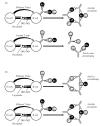
The coexistence of anti-La (SS-B) and anti-Ro (SS-A) autoantibodies in pSS is probably explained by intermolecular spreading of autoimmunity toward different components of the La/Ro ribonucleoprotein (RNP). In order to evaluate the role of the HLA class II phenotype in controlling diversification of this autoantibody response, 80 patients with pSS were typed by polymerase chain reaction sequence-specific oligonucleotide (PCR-SSO) at the HLA class II loci DRB1, DQA1 and DQB1. Serum samples were examined for anti-La and anti-Ro by counterimmunoelectrophoresis and by ELISA using purified recombinant La and 60-kD Ro proteins. Patient sera were classified according to the extent of diversification of the anti-La, anti-Ro response including the presence or absence of precipitating anti-La antibodies. Immunogenic characteristics of these stratified groups were then studied. All patients with pSS, with or without autoantibodies to Ro and La, were found to have at least one of the HLA-DRB1 types DR2, DR3 or DR5. The HLA DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype was primarily associated with a diversified La/Ro RNP response containing precipitating autoantibodies to La (P<0.001); whereas the haplotype HLA DR2-DQA1*0102-DQB1*0602 (DR2-DQ1) was associated with a less diversified La/Ro RNP response containing non-precipitating (restricted epitope) anti-La autoantibodies (P<0.001). Anti-La-positive patients lacking both HLA-DR2 and HLA-DR3 all expressed the HLA-DQA1*0501 allele, which was present at increasing frequency with greater diversification of the anti-La/Ro autoantibody response. The association of distinct HLA haplotypes with different degrees of autoantibody diversification in patients with pSS suggests a model of HLA-restricted presentation of La/Ro peptide determinants to autoreactive helper T cells. We propose that non-precipitating anti-La responses are driven by limited intermolecular help from DR2-DQ1-restricted T helper cells recognizing Ro determinants. On the other hand, we speculate that the more diversified, precipitating anti-La responses obtain more efficient cognate T help from DR3-DQ2-restricted T helper cells recognizing La determinants, where HLA-DQA1*0501 may be a critical determinant for antigen presentation.
Figures
References
-
- Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. - PubMed
-
- Ahearn JM, Provost TT, Dorsch CA, Stevens MB, Bias WB, Arnett FC. The interrelationships of HLA-DR, MB and MT phenotypes, autoantibody expression and clinical features in systemic lupus erythematosus. Arthritis Rheum. 1982;25:1031–40. - PubMed
-
- Hamilton RG, Harley JB, Bias WB, Roebber M, Reichlin M, Hochberg MC, Arnett FC. Two Ro (SS-A) autoantibody responses in systemic lupus erythematosus. Correlation of HLA-DR/DQ specificities with quantitative expression of Ro (SS-A) autoantibody. Arthritis Rheum. 1988;31:496–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

