SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway
- PMID: 9487128
- PMCID: PMC25287
- DOI: 10.1091/mbc.9.3.585
SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway
Abstract
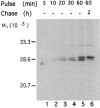
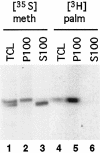
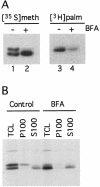
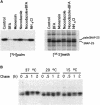
Synaptosomal-associated protein of 25 kDa (SNAP-25) is a palmitoylated membrane protein essential for neurotransmitter release from synaptic terminals. We used neuronal cell lines to study the biosynthesis and posttranslational processing of SNAP-25 to investigate how palmitoylation contributes to the subcellular localization of the protein. SNAP-25 was synthesized as a soluble protein that underwent palmitoylation approximately 20 min after synthesis. Palmitoylation of the protein coincided with its stable membrane association. Treatment of cells with brefeldin A or other disrupters of transport inhibited palmitoylation of newly synthesized SNAP-25 and abolished membrane association. These results demonstrate that the processing of SNAP-25 and its targeting to the plasma membrane depend on an intact transport mechanism along the exocytic pathway. The kinetics of SNAP-25 palmitoylation and membrane association and the sensitivity of these parameters to brefeldin A suggest a novel trafficking pathway for targeting proteins to the plasma membrane. In vitro, SNAP-25 stably associated with membranes was not released from the membrane after chemical deacylation. We propose that palmitoylation of SNAP-25 is required for initial membrane targeting of the protein but that other interactions can maintain membrane association in the absence of fatty acylation.
Figures



Similar articles
-
Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin.Biochem J. 2000 Jan 1;345 Pt 1(Pt 1):145-51. Biochem J. 2000. PMID: 10600650 Free PMC article.
-
Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25.FEBS Lett. 1996 Apr 29;385(1-2):119-23. doi: 10.1016/0014-5793(96)00362-6. FEBS Lett. 1996. PMID: 8641455
-
Characterization of the palmitoylation domain of SNAP-25.J Neurochem. 1997 Nov;69(5):1864-9. doi: 10.1046/j.1471-4159.1997.69051864.x. J Neurochem. 1997. PMID: 9349529
-
GAP-43 as a modulator of G protein transduction in the growth cone.Perspect Dev Neurobiol. 1992;1(1):13-9. Perspect Dev Neurobiol. 1992. PMID: 1364285 Review.
-
Targeting of Specialized Metabolites Biosynthetic Enzymes to Membranes and Vesicles by Posttranslational Palmitoylation: A Mechanism of Non-Conventional Traffic and Secretion of Fungal Metabolites.Int J Mol Sci. 2024 Jan 19;25(2):1224. doi: 10.3390/ijms25021224. Int J Mol Sci. 2024. PMID: 38279221 Free PMC article. Review.
Cited by
-
PI(4,5)P2: signaling the plasma membrane.Biochem J. 2022 Nov 11;479(21):2311-2325. doi: 10.1042/BCJ20220445. Biochem J. 2022. PMID: 36367756 Free PMC article.
-
Ykt6-dependent endosomal recycling is required for Wnt secretion in the Drosophila wing epithelium.Development. 2020 Aug 14;147(15):dev185421. doi: 10.1242/dev.185421. Development. 2020. PMID: 32611603 Free PMC article.
-
Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts.J Neurosci. 2002 Oct 15;22(20):8891-901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. J Neurosci. 2002. PMID: 12388596 Free PMC article.
-
The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediate membrane interactions and recognition by DHHC palmitoyl transferases.Mol Biol Cell. 2009 Mar;20(6):1845-54. doi: 10.1091/mbc.e08-09-0944. Epub 2009 Jan 21. Mol Biol Cell. 2009. PMID: 19158383 Free PMC article.
-
The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells. Regulation by distinct cysteine-rich domains.J Biol Chem. 2005 Jan 14;280(2):1236-40. doi: 10.1074/jbc.M410674200. Epub 2004 Nov 12. J Biol Chem. 2005. PMID: 15542596 Free PMC article.
References
-
- Berthiaume L, Resh M. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. - PubMed
-
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. - PubMed
-
- Duc C, Catsicas S. Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. J Comp Neurol. 1995;356:152–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

