SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway
- PMID: 9487128
- PMCID: PMC25287
- DOI: 10.1091/mbc.9.3.585
SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway
Abstract
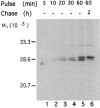
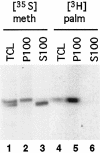
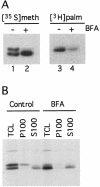
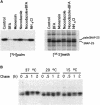
Synaptosomal-associated protein of 25 kDa (SNAP-25) is a palmitoylated membrane protein essential for neurotransmitter release from synaptic terminals. We used neuronal cell lines to study the biosynthesis and posttranslational processing of SNAP-25 to investigate how palmitoylation contributes to the subcellular localization of the protein. SNAP-25 was synthesized as a soluble protein that underwent palmitoylation approximately 20 min after synthesis. Palmitoylation of the protein coincided with its stable membrane association. Treatment of cells with brefeldin A or other disrupters of transport inhibited palmitoylation of newly synthesized SNAP-25 and abolished membrane association. These results demonstrate that the processing of SNAP-25 and its targeting to the plasma membrane depend on an intact transport mechanism along the exocytic pathway. The kinetics of SNAP-25 palmitoylation and membrane association and the sensitivity of these parameters to brefeldin A suggest a novel trafficking pathway for targeting proteins to the plasma membrane. In vitro, SNAP-25 stably associated with membranes was not released from the membrane after chemical deacylation. We propose that palmitoylation of SNAP-25 is required for initial membrane targeting of the protein but that other interactions can maintain membrane association in the absence of fatty acylation.
Figures



References
-
- Berthiaume L, Resh M. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. - PubMed
-
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. - PubMed
-
- Duc C, Catsicas S. Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. J Comp Neurol. 1995;356:152–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

