The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells
- PMID: 9487131
- PMCID: PMC25291
- DOI: 10.1091/mbc.9.3.623
The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells
Abstract
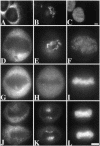
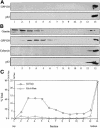
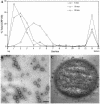
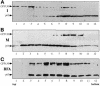
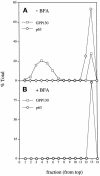
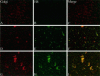
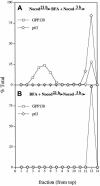
Partitioning of the mammalian Golgi apparatus during cell division involves disassembly at M-phase. Despite the importance of the disassembly/reassembly pathway in Golgi biogenesis, it remains unclear whether mitotic Golgi breakdown in vivo proceeds by direct vesiculation or involves fusion with the endoplasmic reticulum (ER). To test whether mitotic Golgi is fused with the ER, we compared the distribution of ER and Golgi proteins in interphase and mitotic HeLa cells by immunofluorescence microscopy, velocity gradient fractionation, and density gradient fractionation. While mitotic ER appeared to be a fine reticulum excluded from the region containing the spindle-pole body, mitotic Golgi appeared to be dispersed small vesicles that penetrated the area containing spindle microtubules. After cell disruption, M-phase Golgi was recovered in two size classes. The major breakdown product, accounting for at least 75% of the Golgi, was a population of 60-nm vesicles that were completely separated from the ER using velocity gradient separation. The minor breakdown product was a larger, more heterogenously sized, membrane population. Double-label fluorescence analysis of these membranes indicated that this portion of mitotic Golgi also lacked detectable ER marker proteins. Therefore we conclude that the ER and Golgi remain distinct at M-phase in HeLa cells. To test whether the 60-nm vesicles might form from the ER at M-phase as the result of a two-step vesiculation pathway involving ER-Golgi fusion followed by Golgi vesicle budding, mitotic cells were generated with fused ER and Golgi by brefeldin A treatment. Upon brefeldin A removal, Golgi vesicles did not emerge from the ER. In contrast, the Golgi readily reformed from similarly treated interphase cells. We conclude that Golgi-derived vesicles remain distinct from the ER in mitotic HeLa cells, and that mitotic cells lack the capacity of interphase cells for Golgi reemergence from the ER. These experiments suggest that mitotic Golgi breakdown proceeds by direct vesiculation independent of the ER.
Figures

References
-
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995b;82:895–904. - PubMed
-
- Anhquyen L, Steiners JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human alpha 1-antitrypsin. J Biol Chem. 1994;269:7514–7519. - PubMed
-
- Arosond NN, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;21:90–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

