Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine
- PMID: 9488382
- PMCID: PMC108002
- DOI: 10.1128/IAI.66.3.959-965.1998
Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine
Abstract
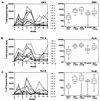
We have analyzed human T-cell responses in parallel with serum immunoglobulin G (IgG) antibody levels after systemic vaccination with the Norwegian group B Neisseria meningitidis outer membrane vesicle (OMV) vaccine. Ten adult volunteers, with no or very low levels of serum IgG antibodies against meningococci, received three doses intramuscularly of the OMV vaccine (at weeks 0, 6, and 46). T-cell proliferation against the OMV vaccine, purified outer membrane proteins (PorA and PorB), and control antigens (Mycobacterium bovis BCG vaccine and tetanus toxoid) was measured by thymidine incorporation of peripheral blood mononuclear cells before and after vaccination. The results showed that vaccination with OMV elicits strong primary and booster T-cell responses specific to OMV as well as the PorA (class 1) protein and significant, but markedly lower, responses against the PorB (class 3) protein. The median responses to OMV and PorA were 26 and 16 times the prevaccination levels, respectively. Most of the vaccinees showed low T-cell responses against OMV and PorA before vaccination, and the maximum T-cell responses to all vaccine antigens were usually obtained after the second vaccine dose. We found a positive correlation between T-cell responses and anti-OMV IgG antibody levels (r = 0.50, P < 0.0001, for OMV and PorA). In addition, we observed a progressive increase in the percentage of CD45R0+ (memory) CD4-positive T cells (P = 0.002). In conclusion, we have shown that the Norwegian OMV vaccine against meningococcal B disease induced antigen-specific T-cell responses, kinetically accompanied by serum IgG responses, and that vaccination increased the proportion of memory T-helper cells.
Figures




References
-
- Ada G L. The immunological principles of vaccination. Lancet. 1990;335:523–526. - PubMed
-
- Akbar A N, Terry L, Timms A, Beverly P C L, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T-cells. J Immunol. 1988;140:2171–2178. - PubMed
-
- Barlow A K, Heckels J E, Clarcke I N. The class 1 outer membrane protein of Neisseria meningitidis: gene sequence and structural and immunological similarities to gonococcal porins. Mol Microbiol. 1989;3:131–139. - PubMed
-
- Bjune G, Høiby E A, Grønnesby J K, Arnesen O, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

