Immunoglobulins to group A streptococcal surface molecules decrease adherence to and invasion of human pharyngeal cells
- PMID: 9488384
- PMCID: PMC108004
- DOI: 10.1128/IAI.66.3.974-979.1998
Immunoglobulins to group A streptococcal surface molecules decrease adherence to and invasion of human pharyngeal cells
Abstract
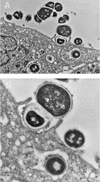
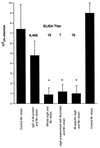
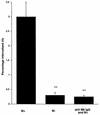
The M protein is one of the most important virulence factors of group A streptococci (Streptococcus pyogenes) and may play an important role in the first steps of streptococcal infection. Since acute pharyngitis is a frequently occurring infectious disease caused by these bacteria, we wished to know whether antibodies to the M protein or other surface components inhibit adherence and internalization of streptococci to pharyngeal cells. We investigated the role of whole human secretory immunoglobulin A (sIgA), M6 protein-specific sIgA, and M6 protein-specific serum IgG in the inhibition of streptococcal adherence and internalization to cultured human pharyngeal cells. S. pyogenes D471, which produces a type 6 M protein (M+), and its isogenic M-negative (M-) derivative JRS75 were tested. Purified whole sIgA, M protein-specific sIgA, and sIgA preabsorbed with M protein were able to decrease significantly the adherence of streptococci to pharyngeal cells. Purified IgG against the M6 protein did not diminish the attachment of streptococci to the pharyngeal cells but did reduce internalization. Thus, our data suggest that secretory IgA may play a key role in preventing streptococcal infection at mucosal surfaces by blocking adherence while affinity-purified anti-M protein-specific IgG blocks epitopes responsible for invasion.
Figures
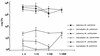
References
-
- Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. - PubMed
-
- Bessen D, Fischetti V A. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990;145:1251–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

