Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition
- PMID: 9488398
- PMCID: PMC108018
- DOI: 10.1128/IAI.66.3.1076-1081.1998
Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition
Abstract
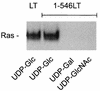
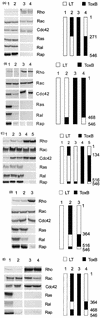
Clostridium sordellii lethal toxin is a member of the family of large clostridial cytotoxins that glucosylate small GTPases. In contrast to Clostridium difficile toxins A and B, which exclusively modify Rho subfamily proteins, C. sordellii lethal toxin also glucosylates Ras subfamily proteins. By deletion analysis and construction of chimeric fusion proteins of C. sordellii lethal toxin and C. difficile toxin B, we localized the enzyme activity of the lethal toxin to the N terminus of the holotoxin and identified the region involved in protein substrate specificity. The toxin fragment of the N-terminal 546 amino acid residues of C. sordellii lethal toxin glucosylated Rho and Ras subfamily proteins, as the holotoxin did. Deletion of a further 30 amino acid residues from the C terminus of this active fragment drastically reduced glucotransferase activity and blocked glucohydrolase activity. Exchange of amino acid residues 364 through 516 of lethal toxin for those in the active toxin B fragment (1 to 546) allowed glucosylation of Ras subfamily proteins. In contrast, the chimera with amino acids 1 to 364 from toxin B, 365 to 468 from lethal toxin, and 469 to 546 from toxin B exhibited markedly reduced modification of Ras subfamily proteins, whereas modification of Rac and Cdc42 was hardly changed. The data indicate that the region of amino acid residues 364 through 516 primarily defines the substrate specificity of C. sordellii lethal toxin.
Figures





References
-
- Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. - PubMed
-
- Baldacini O, Girardot R, Green G A, Rihn B, Monteil H. Comparative study of immunological properties and cytotoxic effects of Clostridium difficile toxin B and Clostridium sordelii toxin L. Toxicon. 1992;30:129–140. - PubMed
-
- Barroso L A, Moncrief J S, Lyerly D M, Wilkins T D. Mutagenesis of the Clostridium difficile toxin B gene and effect on cytotoxic activity. Microb Pathog. 1994;16:297–303. - PubMed
-
- Bette P, Oksche A, Mauler F, Eichel-Streiber C, Popoff M R, Habermann E. A comparative biochemical, pharmacological and immunological study of Clostridium novyi α-toxin, C. difficile toxin B and C. sordellii lethal toxin. Toxicon. 1991;29:877–887. - PubMed
-
- Bongaerts G P A, Lyerly D M. Role of toxins A and B in the pathogenesis of Clostridium difficile disease. Microb Pathog. 1994;17:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

