Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract
- PMID: 9488417
- PMCID: PMC108037
- DOI: 10.1128/IAI.66.3.1225-1232.1998
Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract
Abstract
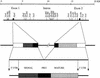
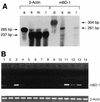
One component of host defense at mucosal surfaces appears to be epithelium-derived peptides with antimicrobial activity called defensins. Human beta-defensin 1 (hBD-1) represents the first member of the beta-defensin family isolated from humans and has been implicated in the pathogenesis of cystic fibrosis. We describe in this report the isolation and characterization of a murine homolog of hBD-1 called mouse beta-defensin 1 (mBD-1). The predicted amino acid sequence shows the hallmark features of other known epithelial beta-defensins, including the ordered array of six cysteine residues. Analysis of a genomic clone of mBD-1 revealed two exons separated by a 15-kb intron. By use of fluorescence in situ hybridization, the mBD-1 gene was localized at the proximal portion of chromosome 8, the site where mouse alpha-defensins are found. Lysates from cells transfected with the mBD-1 cDNA showed antibacterial activity against gram-positive and gram-negative bacteria. mBD-1 transcripts were found in kidney, liver, and female reproductive organ tissues. In the airways, mBD-1 is expressed diffusely throughout the epithelial cells of the large proximal airways with less expression in the small distal airways and no expression in alveolar cells. The present study demonstrates that a beta-defensin potentially homologous to human beta-defensin 1 is present in the respiratory system and other mucosal surfaces in mice.
Figures





References
-
- Bensch K W, Raida M, Magert H-J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

