Gal4p-mediated chromatin remodeling depends on binding site position in nucleosomes but does not require DNA replication
- PMID: 9488435
- PMCID: PMC108833
- DOI: 10.1128/MCB.18.3.1201
Gal4p-mediated chromatin remodeling depends on binding site position in nucleosomes but does not require DNA replication
Abstract
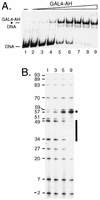
Biochemical studies have demonstrated decreased binding of various proteins to DNA in nucleosome cores as their cognate sites are moved from the edge of the nucleosome to the pseudodyad (center). However, to date no study has addressed whether this structural characteristic of nucleosomes modulates the function of a transcription factor in living cells, where processes of DNA replication and chromatin modification or remodeling could significantly affect factor binding. Using a sensitive, high-resolution methyltransferase assay, we have monitored the ability of Gal4p in vivo to interact with a nucleosome at positions that are known to be inaccessible in nucleosome cores in vitro. Gal4p efficiently bound a single cognate site (UASG) centered at 41 bp from the edge of a positioned nucleosome, perturbing chromatin structure and inducing transcription. DNA binding and chromatin perturbation accompanying this interaction also occurred in the presence of hydroxyurea, indicating that DNA replication is not necessary for Gal4p-mediated nucleosome disruption. These data extend previous studies, which demonstrated DNA replication-independent chromatin remodeling, by showing that a single dimer of Gal4p, without the benefit of cooperative interactions that occur at complex wild-type promoters, is competent for invasion of a preestablished nucleosome. When the UASG was localized at the nucleosomal pseudodyad, relative occupancy by Gal4p, nucleosome disruption, and transcriptional activation were substantially compromised. Therefore, despite the increased nucleosome binding capability of Gal4p in cells, the precise translational position of a factor binding site in one nucleosome in an array can affect the ability of a transcriptional regulator to overcome the repressive influence of chromatin.
Figures








References
-
- Archer T K, Cordingley M G, Marsaud V, Richard-Foy H, Hager G L. Steroid transactivation at a promoter organized in a specifically-positioned array of nucleosomes. In: Gustafsson J A, Eriksson H, Carlstedt-Duke J, editors. Steroid/thyroid hormone receptor family and gene regulation. Berlin, Germany: Berkhauser Verlag, AG; 1989. pp. 221–238.
-
- Barton M C, Emerson B M. Regulated expression of the β-globin gene locus in synthetic nuclei. Genes Dev. 1994;8:2453–2465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
