Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis
- PMID: 9488438
- PMCID: PMC108836
- DOI: 10.1128/MCB.18.3.1236
Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis
Abstract
The human CAN gene was first identified as a target of t(6;9)(p23;q34), associated with acute myeloid leukemia and myelodysplastic syndrome, which results in the expression of a DEK-CAN fusion gene. CAN, also called NUP214, is a nuclear pore complex (NPC) protein that contains multiple FG-peptide sequence motifs. It interacts at the NPC with at least two other proteins, the nucleoporin NUP88 and hCRM1 (exportin 1), which was recently shown to function as a nuclear export receptor. Depletion of CAN in knockout mouse embryonic cells results in cell cycle arrest in G2, followed by inhibition of nuclear protein import and a block of mRNA export. We overexpressed CAN and DEK-CAN in U937 myeloid precursor cells. DEK-CAN expression did not interfere with terminal myeloid differentiation of U937 cells, whereas CAN-overexpressing cells arrested in G0, accumulated mRNA in their nuclei, and died in an apoptotic manner. Interestingly, we found that hCRM1 and import factor p97/importin beta colocalized with the ectopically expressed CAN protein, resulting in depletion of both factors from the NPC. Overexpression of the C-terminal FG-repeat region of CAN, which contains the binding site for hCRM1, caused sequestering of hCRM1 in the nucleoplasm and was sufficient to inhibit cell growth and to induce apoptosis. These results confirm that CAN plays a crucial role in nucleocytoplasmic transport and imply an essential role for hCRM1 in cell growth and survival.
Figures




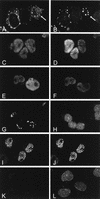
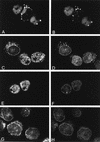


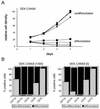
References
-
- Adam S A, Gerace L. Cytosolic proteins that specifically bind nuclear localization signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
-
- Amberg D C, Goldstein A L, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. - PubMed
-
- Boise L H, González-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nuñez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
