Simian virus 40 large T antigen interacts with human TFIIB-related factor and small nuclear RNA-activating protein complex for transcriptional activation of TATA-containing polymerase III promoters
- PMID: 9488448
- PMCID: PMC108846
- DOI: 10.1128/MCB.18.3.1331
Simian virus 40 large T antigen interacts with human TFIIB-related factor and small nuclear RNA-activating protein complex for transcriptional activation of TATA-containing polymerase III promoters
Abstract
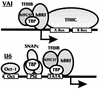
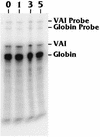
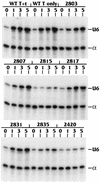
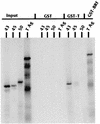
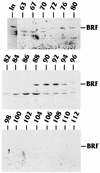
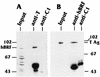
The TATA-binding protein (TBP) is common to the basal transcription factors of all three RNA polymerases, being associated with polymerase-specific TBP-associated factors (TAFs). Simian virus 40 large T antigen has previously been shown to interact with the TBP-TAFII complexes, TFIID (B. Damania and J. C. Alwine, Genes Dev. 10:1369-1381, 1996), and the TBP-TAFI complex, SL1 (W. Zhai, J. Tuan, and L. Comai, Genes Dev. 11: 1605-1617, 1997), and in both cases these interactions are critical for transcriptional activation. We show a similar mechanism for activation of the class 3 polymerase III (pol III) promoter for the U6 RNA gene. Large T antigen can activate this promoter, which contains a TATA box and an upstream proximal sequence element but cannot activate the TATA-less, intragenic VAI promoter (a class 2, pol III promoter). Mutants of large T antigen that cannot activate pol II promoters also fail to activate the U6 promoter. We provide evidence that large T antigen can interact with the TBP-containing pol III transcription factor human TFIIB-related factor (hBRF), as well as with at least two of the three TAFs in the pol III-specific small nuclear RNA-activating protein complex (SNAPc). In addition, we demonstrate that large T antigen can cofractionate and coimmunoprecipitate with the hBRF-containing complex TFIIIB derived from HeLa cells infected with a recombinant adenovirus which expresses large T antigen. Hence, similar to its function with pol I and pol II promoters, large T antigen interacts with TBP-containing, basal pol III transcription factors and appears to perform a TAF-like function.
Figures

Similar articles
-
Simian virus 40 large T antigen stabilizes the TATA-binding protein-TFIIA complex on the TATA element.Mol Cell Biol. 1998 Jul;18(7):3926-35. doi: 10.1128/MCB.18.7.3926. Mol Cell Biol. 1998. PMID: 9632777 Free PMC article.
-
Structure-function analysis of the human TFIIB-related factor II protein reveals an essential role for the C-terminal domain in RNA polymerase III transcription.Mol Cell Biol. 2005 Nov;25(21):9406-18. doi: 10.1128/MCB.25.21.9406-9418.2005. Mol Cell Biol. 2005. PMID: 16227591 Free PMC article.
-
Mechanism of selective recruitment of RNA polymerases II and III to snRNA gene promoters.Genes Dev. 2018 May 1;32(9-10):711-722. doi: 10.1101/gad.314245.118. Epub 2018 May 21. Genes Dev. 2018. PMID: 29785964 Free PMC article.
-
Biochemistry and structural biology of transcription factor IID (TFIID).Annu Rev Biochem. 1996;65:769-99. doi: 10.1146/annurev.bi.65.070196.004005. Annu Rev Biochem. 1996. PMID: 8811195 Review.
-
Regulation of snRNA gene expression by the Drosophila melanogaster small nuclear RNA activating protein complex (DmSNAPc).Crit Rev Biochem Mol Biol. 2011 Feb;46(1):11-26. doi: 10.3109/10409238.2010.518136. Epub 2010 Oct 6. Crit Rev Biochem Mol Biol. 2011. PMID: 20925482 Review.
Cited by
-
A kinase activity associated with simian virus 40 large T antigen phosphorylates upstream binding factor (UBF) and promotes formation of a stable initiation complex between UBF and SL1.Mol Cell Biol. 1999 Apr;19(4):2791-802. doi: 10.1128/MCB.19.4.2791. Mol Cell Biol. 1999. PMID: 10082545 Free PMC article.
-
Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain.Clin Microbiol Rev. 2012 Jul;25(3):471-506. doi: 10.1128/CMR.05031-11. Clin Microbiol Rev. 2012. PMID: 22763635 Free PMC article. Review.
-
Single-Cell Transcriptomics Reveals a Heterogeneous Cellular Response to BK Virus Infection.J Virol. 2021 Feb 24;95(6):e02237-20. doi: 10.1128/JVI.02237-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361432 Free PMC article.
-
Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model.Am J Pathol. 2000 Sep;157(3):805-13. doi: 10.1016/S0002-9440(10)64594-4. Am J Pathol. 2000. PMID: 10980120 Free PMC article.
-
Simian virus 40 large T antigen stabilizes the TATA-binding protein-TFIIA complex on the TATA element.Mol Cell Biol. 1998 Jul;18(7):3926-35. doi: 10.1128/MCB.18.7.3926. Mol Cell Biol. 1998. PMID: 9632777 Free PMC article.
References
-
- Beard P, Bruggmann H. Control of transcription in vitro from simian virus 40 promoters by proteins from viral minichromosomes. Curr Top Microbiol Immunol. 1989;144:47–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
