Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku-DNA-dependent protein kinase complex
- PMID: 9488450
- PMCID: PMC108848
- DOI: 10.1128/MCB.18.3.1349
Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku-DNA-dependent protein kinase complex
Abstract
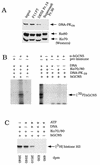
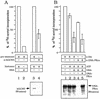
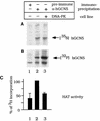
GCN5, a putative transcriptional adapter in humans and yeast, possesses histone acetyltransferase (HAT) activity which has been linked to GCN5's role in transcriptional activation in yeast. In this report, we demonstrate a functional interaction between human GCN5 (hGCN5) and the DNA-dependent protein kinase (DNA-PK) holoenzyme. Yeast two-hybrid screening detected an interaction between the bromodomain of hGCN5 and the p70 subunit of the human Ku heterodimer (p70-p80), which is the DNA-binding component of DNA-PK. Interaction between intact hGCN5 and Ku70 was shown biochemically using recombinant proteins and by coimmunoprecipitation of endogenous proteins following chromatography of HeLa nuclear extracts. We demonstrate that the catalytic subunit of DNA-PK phosphorylates hGCN5 both in vivo and in vitro and, moreover, that the phosphorylation inhibits the HAT activity of hGCN5. These findings suggest a possible regulatory mechanism of HAT activity.
Figures





References
-
- Abmayr S M, Workman J L. Preparation of nuclear and cytoplasmic extracts from mammalian cells, units 12.1.1–12.1.9. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1993. - PubMed
-
- Anderson C W. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993;18:433–437. - PubMed
-
- Arany Z, Sellers W R, Livingston D M, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. . (Letter.) - PubMed
-
- Bannister A, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
