The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor
- PMID: 9488452
- PMCID: PMC108850
- DOI: 10.1128/MCB.18.3.1369
The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor
Abstract
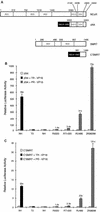
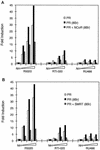
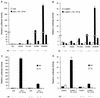
Previously, we defined a novel class of ligands for the human progesterone receptor (PR) which function as mixed agonists. These compounds induce a conformational change upon binding the receptor that is different from those induced by agonists and antagonists. This establishes a correlation between the structure of a ligand-receptor complex and its transcriptional activity. In an attempt to define the cellular components which distinguish between different ligand-induced PR conformations, we have determined, by using a mammalian two-hybrid assay, that the nuclear receptor corepressor (NCoR) and the silencing mediator for retinoid and thyroid hormone receptor (SMRT) differentially associate with PR depending upon the class of ligand bound to the receptor. Specifically, we observed that the corepressors preferentially associate with antagonist-occupied PR and that overexpression of these corepressors suppresses the partial agonist activity of antagonist-occupied PR. Binding studies performed in vitro, however, reveal that recombinant SMRT can interact with PR in a manner which is not influenced by the nature of the bound ligand. Thus, the inability of SMRT or NCoR to interact with agonist-activated PR when assayed in vivo may relate more to the increased affinity of PR for coactivators, with a subsequent displacement of corepressors, than to an inherent low affinity for the corepressor proteins. Previous work from other groups has shown that 8-bromo-cyclic AMP (8-bromo-cAMP) can convert the PR antagonist RU486 into an agonist and, additionally, can potentiate the transcriptional activity of agonist-bound PR. In this study, we show that exogenous expression of NCoR or SMRT suppresses all 8-bromo-cAMP-mediated potentiation of PR transcriptional activity. Further analysis revealed that 8-bromo-cAMP addition decreases the association of NCoR and SMRT with PR. Thus, we propose that 8-bromo-cAMP-mediated potentiation of PR transcriptional activity is due, at least in part, to a disruption of the interaction between PR and the corepressors NCoR and SMRT. Cumulatively, these results suggest that NCoR and SMRT expression may play a pivotal role in PR pharmacology.
Figures




References
-
- Allan G F, Leng X, Tsai S-T, Weigel N L, Edwards D P, Tsai M-J, O’Malley B W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267:19513–19520. - PubMed
-
- Allan G F, Lombardi E, Haynes-Johnson D, Palmer S, Kiddoe M, Kraft P, Campen C, Rybczynski P, Combs D W, Phillips A. Induction of a novel conformation in the progesterone receptor by ZK299 involves a defined region of the carboxyl-terminal tail. Mol Endocrinol. 1996;10:1206–1213. - PubMed
-
- Baniahmad A, Steiner C, Kohne A C, Renkawitz R. Molecular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. EMBO J. 1990;61:505–514. - PubMed
-
- Beck C A, Weigel N L, Edwards D P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
