Reinitiation of DNA synthesis and cell division in senescent human fibroblasts by microinjection of anti-p53 antibodies
- PMID: 9488478
- PMCID: PMC108876
- DOI: 10.1128/MCB.18.3.1611
Reinitiation of DNA synthesis and cell division in senescent human fibroblasts by microinjection of anti-p53 antibodies
Abstract
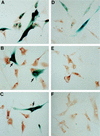
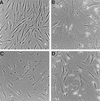
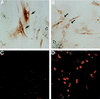
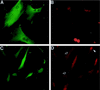
In human fibroblasts, growth arrest at the end of the normal proliferative life span (induction of senescence) is dependent on the activity of the tumor suppressor protein p53. In contrast, once senescence has been established, it is generally accepted that reinitiation of DNA synthesis requires loss of multiple suppressor pathways, for example, by expression of Simian virus 40 (SV40) large T antigen, and that even this will not induce complete cell cycle traverse. Here we have used microinjection of monoclonal antibodies to the N terminus of p53, PAb1801 and DO-1, to reinvestigate the effect of blocking p53 function in senescent human fibroblasts. Unexpectedly, we found that both antibodies induce senescent cells to reenter S phase almost as efficiently as SV40, accompanied by a reversion to the "young" morphology. Furthermore, this is followed by completion of the cell division cycle, as shown by the appearance of mitoses, and by a four- to fivefold increase in cell number 9 days after injection. Immunofluorescence analysis showed that expression of the p53-inducible cyclin/kinase inhibitor p21sdi1/WAF1 was greatly diminished by targeting p53 with either PAb1801 or DO-1 but remained high and, moreover, still p53 dependent in cells expressing SV40 T antigen. As previously observed for induction, the maintenance of fibroblast senescence therefore appears to be critically dependent on functional p53. We suggest that the previous failure to observe this by using SV40 T-antigen mutants to target p53 was most probably due to incomplete abrogation of p53 function.
Figures




References
-
- Abarzua P, LoSardo J E, Gubler M L, Neri A. Microinjection of monoclonal antibody PAb421 into human SW480 colorectal carcinoma cells restores the transcription activation function to mutant p53. Cancer Res. 1995;55:3490–3494. - PubMed
-
- Bacchetti S. Telomere dynamics and telomerase activity in cell senescence and cancer. Cell Dev Biol. 1996;7:31–39.
-
- Banks L, Matlashewski G, Crawford L. Isolation of human p53 specific monoclonal antibodies and their use in the study of human p53 expression. Eur J Biochem. 1986;159:529–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
