The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex
- PMID: 9488488
- PMCID: PMC108886
- DOI: 10.1128/MCB.18.3.1711
The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex
Abstract
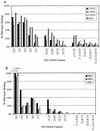
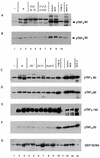
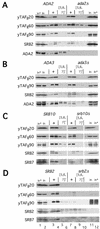
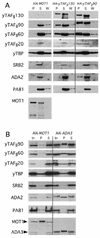
The Gcn4p activation domain contains seven clusters of hydrophobic residues that make additive contributions to transcriptional activation in vivo. We observed efficient binding of a glutathione S-transferase (GST)-Gcn4p fusion protein to components of three different coactivator complexes in Saccharomyces cerevisiae cell extracts, including subunits of transcription factor IID (TFIID) (yeast TAFII20 [yTAFII20], yTAFII60, and yTAFII90), the holoenzyme mediator (Srb2p, Srb4p, and Srb7p), and the Adap-Gcn5p complex (Ada2p and Ada3p). The binding to these coactivator subunits was completely dependent on the hydrophobic clusters in the Gcn4p activation domain. Alanine substitutions in single clusters led to moderate reductions in binding, double-cluster substitutions generally led to greater reductions in binding than the corresponding single-cluster mutations, and mutations in four or more clusters reduced binding to all of the coactivator proteins to background levels. The additive effects of these mutations on binding of coactivator proteins correlated with their cumulative effects on transcriptional activation by Gcn4p in vivo, particularly with Ada3p, suggesting that recruitment of these coactivator complexes to the promoter is a cardinal function of the Gcn4p activation domain. As judged by immunoprecipitation analysis, components of the mediator were not associated with constituents of TFIID and Adap-Gcn5p in the extracts, implying that GST-Gcn4p interacted with the mediator independently of these other coactivators. Unexpectedly, a proportion of Ada2p coimmunoprecipitated with yTAFII90, and the yTAFII20, -60, and -90 proteins were coimmunoprecipitated with Ada3p, revealing a stable interaction between components of TFIID and the Adap-Gcn5p complex. Because GST-Gcn4p did not bind specifically to highly purified TFIID, Gcn4p may interact with TFIID via the Adap-Gcn5p complex or some other adapter proteins. The ability of Gcn4p to interact with several distinct coactivator complexes that are physically and genetically linked to TATA box-binding protein can provide an explanation for the observation that yTAFII proteins are dispensable for activation by Gcn4p in vivo.
Figures


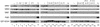



References
-
- Apone L M, Virbasius C M, Reese J C, Green M R. Yeast TAF(II)90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. - PubMed
-
- Barlev N A, Candau R, Wang L, Darpino P, Silverman N, Berger S L. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
