Novel mechanisms of E2F induction by BK virus large-T antigen: requirement of both the pRb-binding and the J domains
- PMID: 9488491
- PMCID: PMC108889
- DOI: 10.1128/MCB.18.3.1746
Novel mechanisms of E2F induction by BK virus large-T antigen: requirement of both the pRb-binding and the J domains
Abstract
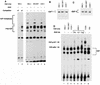
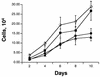
E2F activity is regulated in part by the retinoblastoma family of tumor suppressor proteins. Viral oncoproteins, such as simian virus 40 (SV40) large-T antigen (TAg), adenovirus E1A, and human papillomavirus E7, can disrupt the regulation of cellular proliferation by binding to pRb family members and dissociating E2F-pRb family protein complexes. BK virus (BKV), which infects a large percentage of the human population and has been associated with a variety of human tumors, encodes a TAg homologous to SV40 TAg. It has been shown that BKV TAg, when expressed at low levels, does not detectably bind to pRb family members, yet it induces a serum-independent phenotype and causes a decrease in the overall levels of pRb family proteins. The experiments presented in this report show that, despite the lack of TAg-pRb interactions, BKV TAg can induce transcriptionally active E2F and that this induction does in fact require an intact pRb-binding domain as well as an intact J domain. In addition, E2F-pRb family member complexes can be detected in both BKV and SV40 TAg-expressing cells. These results suggest the presence of alternate cellular mechanisms for the release of E2F in addition to the well-established model for TAg-pRb interactions. These results also emphasize a role for BKV TAg in the deregulation of cellular proliferation, which may ultimately contribute to neoplasia.
Figures




References
-
- Arthur R R, Shah K V, Baust S J, Santos G W, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. - PubMed
-
- Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991;65:1063–1072. - PubMed
-
- Bandara L R, Adamczewski J P, Hunt T, La Thangue N B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991;352:249–251. - PubMed
-
- Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
