Formation and function of the Rbl2p-beta-tubulin complex
- PMID: 9488492
- PMCID: PMC108890
- DOI: 10.1128/MCB.18.3.1757
Formation and function of the Rbl2p-beta-tubulin complex
Abstract
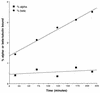
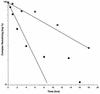
The yeast protein Rbl2p suppresses the deleterious effects of excess beta-tubulin as efficiently as does alpha-tubulin. Both in vivo and in vitro, Rbl2p forms a complex with beta-tubulin that does not contain alpha-tubulin, thus defining a second pool of beta-tubulin in the cell. Formation of the complex depends upon the conformation of beta-tubulin. Newly synthesized beta-tubulin can bind to Rbl2p before it binds to alpha-tubulin. Rbl2p can also bind beta-tubulin from the alpha/beta-tubulin heterodimer, apparently by competing with alpha-tubulin. The Rbl2p-beta-tubulin complex has a half-life of approximately 2.5 h and is less stable than the alpha/beta-tubulin heterodimer. The results of our experiments explain both how excess Rbl2p can rescue cells overexpressing beta-tubulin and how it can be deleterious in a wild-type background. They also suggest that the Rbl2p-beta-tubulin complex is part of a cellular mechanism for regulating the levels and dimerization of tubulin chains.
Figures


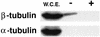
References
-
- Archer J E, Vega L R, Solomon F. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. - PubMed
-
- Campo R, Fontalba A, Sanchez L, Zabala J. A 14kDa release factor is involved in GTP-dependent beta-tubulin folding. FEBS Lett. 1994;353:162–166. - PubMed
-
- Compton, K., and F. Solomon. Unpublished results.
-
- Detrich H W, Williams R C. Reversible dissociation of the alpha-beta dimer of tubulin from bovine brain. Biochemistry. 1978;17:3900–3907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
