Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures
- PMID: 9489022
- PMCID: PMC35135
- DOI: 10.1104/pp.116.2.755
Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures
Abstract
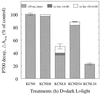
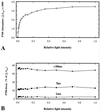
Light-induced damage to photosystem I (PSI) was studied during low-light illumination of barley (Hordeum vulgare L.) at chilling temperatures. A 4-h illumination period induced a significant inactivation of PSI electron transport activity. Flash-induced P700 absorption decay measurements revealed progressive damage to (a) the iron-sulfur clusters FA and FB, (b) the iron-sulfur clusters FA, FB, and FX, and (c) the phylloquinone A1 and the chlorophyll AO or P700 of the PSI electron acceptor chain. Light-induced PSI damage was also evidenced by partial degradation of the PSI-A and PSI-B proteins and was correlated with the appearance of smaller proteins. Aggravated photodamage was observed upon illumination of barley leaves infiltrated with KCN, which inhibits Cu,Zn-superoxide dismutase and ascorbate peroxidase. This indicates that the photodamage of PSI in barley observed during low-light illumination at chilling temperatures arises because the defense against active oxygen species by active oxygen-scavenging enzymes is insufficient at these specific conditions. The data obtained demonstrate that photoinhibition of PSI at chilling temperatures is an important phenomenon in a cold-tolerant plant species.
Figures






References
-
- Anderson JM, Andersson B. The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci. 1988;13:351–355. - PubMed
-
- Andersson B, Styring S. Photosystem II: molecular organisation, function, and acclimation. Curr Top Bioenerg. 1991;16:1–81.
-
- Aro EM, Hundal T, Carlberg I, Andersson B. In vitro studies on light-induced inhibition of photosystem II and D1-protein degradation at low temperatures. Biochim Biophys Acta. 1990;1019:269–275.
-
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

