Plastid ontogeny during petal development in Arabidopsis
- PMID: 9489024
- PMCID: PMC35139
- DOI: 10.1104/pp.116.2.797
Plastid ontogeny during petal development in Arabidopsis
Abstract
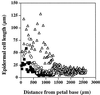
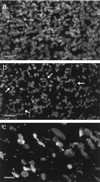
Imaging of chlorophyll autofluorescence by confocal microscopy in intact whole petals of Arabidopsis thaliana has been used to analyze chloroplast development and redifferentiation during petal development. Young petals dissected from unopened buds contained green chloroplasts throughout their structure, but as the upper part of the petal lamina developed and expanded, plastids lost their chlorophyll and redifferentiated into leukoplasts, resulting in a white petal blade. Normal green chloroplasts remained in the stalk of the mature petal. In epidermal cells the chloroplasts were normal and green, in stark contrast with leaf epidermal cell plastids. In addition, the majority of these chloroplasts had dumbbell shapes, typical of dividing chloroplasts, and we suggest that the rapid expansion of petal epidermal cells may be a trigger for the initiation of chloroplast division. In petals of the Arabidopsis plastid division mutant arc6, the conversion of chloroplasts into leukoplasts was unaffected in spite of the greatly enlarged size and reduced number of arc6 chloroplasts in cells in the petal base, resulting in few enlarged leukoplasts in cells from the white lamina of arc6 petals.
Figures






References
-
- Brett DW, Sommerard AP. Ultrastructural development of plastids in the epidermis and starch layer of glossy Ranunculus petals. Ann Bot. 1986;58:903–910.
-
- Dupree P, Pwee K-H, Gray JC. Expression of photosynthetic gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J. 1991;1:115–120.
-
- Falk H. Chromoplasts of Tropaeolum majus L.: structure and development. Planta. 1976;128:15–22. - PubMed
-
- Kirk JTO, Tilney-Bassett RAE (1978) The Plastids. Elsevier/North Holland Biomedical Press, Amsterdam, The Netherlands
-
- Lawrence SD, Cline K, Moore GA. Chromoplast development in ripening tomato fruit: identification of cDNAs for chromoplast-targeted proteins and characterisation of a cDNA encoding a plastid-localised low-molecular weight heat shock protein. Plant Mol Biol. 1997;33:483–492. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

